My follow up question is will the molecules of carbon dioxide feel a force when dissolved in water from the dipoles of water since from what I understand stand from the replies charges will still be present a either side of the molecule. However, the low solubility of apolar CO2 in polar water negatively impacts the electrochemical process, especially mass transport. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. In this article, we will discuss the concept of polarity in chemistry, the difference between BTW, it should be the most adapted to SE. Photo: Jynto via Wikimedia Commons, Public Domain. You can see in the above image that because of electronegativity difference, the partial positive charge (+) appears on the Carbon atom (C) and partial negative charge (-) appears on the Oxygen atoms (O). Likewise, if a molecule does not have regions of positive and negative charge, its considered nonpolar. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. WebMind a CO2, mind a H2O kt polris ktst tartalmaz. 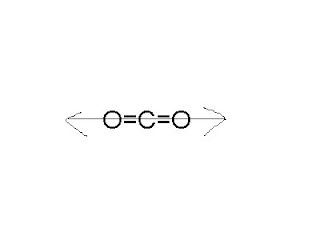 In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. The polarity of water has an enormous impact on its physical and chemical properties. WebThe Greek letter delta indicates "partially".
In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. The polarity of water has an enormous impact on its physical and chemical properties. WebThe Greek letter delta indicates "partially".  The problem you posted earlier was CO2, so you assume that the total charge is zero. (Note: If you want to know the steps of drawing the CO2 lewis dot structure, then visit this article: CO2 lewis structure). Is CF4 Ionic/Polar/Non Polar. Stated plainly, the electronic displacement from A atom towards the two B atoms (or the other way around if A is more electronegative than B atoms) must be symmetric with respect to the central atom, ending up with a molecule without a net electric dipole. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang 4.4: Polar and Non-polar Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. There is also something of a "bonus". Linear B-A-B molecules cannot be polar. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. WebDiffusion NATURAL Tendency for molecules of a substance to fill an available space. Which hydrocarbon group is the least reactive, why? Is Carbon Dioxide (CO2) Polar Or Nonpolar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Why is CO2 a Nonpolar molecule? Due to their different electronegativity, carbon and oxygen do not share the same number of electrons. The polar bonds in the bent H2O molecule result in a net dipole moment, so H2O is polar. WebAtom polar terjadi ketika atom yang berbeda terikat dalam molekul, seperti karbon dioksida (CO2) dan air (H2O), dimana tarikan atom tertentu menyebabkan distribusi elektron menjadi tidak merata. IONIC. For example, if you want to mix an ionic compound or polar compound in an organic solvent, you may be able to dissolve it in ethanol (polar, but not by a lot). (And Why? Really, who is who? The charge at the end of the equation CO2^-2 belongs to the whole compound. H2O2 molecule is a nonplanar and asymmetric molecule. Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbons electron density the exact same way. Understanding the polarity of molecules is essential in various fields of chemistry, including organic chemistry, biochemistry, and material science. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. It only takes a minute to sign up. The physical properties of water and carbon dioxide are affected by their polarities. Which Is The Most Reactive Element In The Periodic Table? What type of bond is formed between two atoms if the difference in electronegativities is small? Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. I know that the symmetry argument is not very satisfying the first times it is brought to the table, but once you grasp the idea, it became so powerful and ubiquitous that it is always the first thing you look for in a new problem! Lucky Block New Cryptocurrency with $750m+ Market Cap Lists on LBank, Carbon dioxide is considered a nonpolar molecule, Photo: Richard Wheeler (Zephyris) via Wikimedia Commons, CC-BY-SA 3.0. }
Use MathJax to format equations. border: #dbdbdb 0px solid;
The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. }
We therefore have a "hand in glove" situation where the water has its quadrupole aligned with the carbon dioxide quadrupole but the charges oppositely distributed, enabling every atom of the water molecule to simultaneously attract an appropriate atom from the carbon dioxide. Because of this, there are no positive and negative poles of charges on the overall molecule of CO2. #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? so, is ccl4 polar or nonpolar? Assertion : CO 2 molecule is non-polar while SO 2 is polar. [insert object name]) in real life to get things done. All the bonds within a molecule have to be considered when determining the polarity of a molecule.
The problem you posted earlier was CO2, so you assume that the total charge is zero. (Note: If you want to know the steps of drawing the CO2 lewis dot structure, then visit this article: CO2 lewis structure). Is CF4 Ionic/Polar/Non Polar. Stated plainly, the electronic displacement from A atom towards the two B atoms (or the other way around if A is more electronegative than B atoms) must be symmetric with respect to the central atom, ending up with a molecule without a net electric dipole. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang 4.4: Polar and Non-polar Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. There is also something of a "bonus". Linear B-A-B molecules cannot be polar. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. WebDiffusion NATURAL Tendency for molecules of a substance to fill an available space. Which hydrocarbon group is the least reactive, why? Is Carbon Dioxide (CO2) Polar Or Nonpolar? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Why is CO2 a Nonpolar molecule? Due to their different electronegativity, carbon and oxygen do not share the same number of electrons. The polar bonds in the bent H2O molecule result in a net dipole moment, so H2O is polar. WebAtom polar terjadi ketika atom yang berbeda terikat dalam molekul, seperti karbon dioksida (CO2) dan air (H2O), dimana tarikan atom tertentu menyebabkan distribusi elektron menjadi tidak merata. IONIC. For example, if you want to mix an ionic compound or polar compound in an organic solvent, you may be able to dissolve it in ethanol (polar, but not by a lot). (And Why? Really, who is who? The charge at the end of the equation CO2^-2 belongs to the whole compound. H2O2 molecule is a nonplanar and asymmetric molecule. Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbons electron density the exact same way. Understanding the polarity of molecules is essential in various fields of chemistry, including organic chemistry, biochemistry, and material science. If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. It only takes a minute to sign up. The physical properties of water and carbon dioxide are affected by their polarities. Which Is The Most Reactive Element In The Periodic Table? What type of bond is formed between two atoms if the difference in electronegativities is small? Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. I know that the symmetry argument is not very satisfying the first times it is brought to the table, but once you grasp the idea, it became so powerful and ubiquitous that it is always the first thing you look for in a new problem! Lucky Block New Cryptocurrency with $750m+ Market Cap Lists on LBank, Carbon dioxide is considered a nonpolar molecule, Photo: Richard Wheeler (Zephyris) via Wikimedia Commons, CC-BY-SA 3.0. }
Use MathJax to format equations. border: #dbdbdb 0px solid;
The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. }
We therefore have a "hand in glove" situation where the water has its quadrupole aligned with the carbon dioxide quadrupole but the charges oppositely distributed, enabling every atom of the water molecule to simultaneously attract an appropriate atom from the carbon dioxide. Because of this, there are no positive and negative poles of charges on the overall molecule of CO2. #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? so, is ccl4 polar or nonpolar? Assertion : CO 2 molecule is non-polar while SO 2 is polar. [insert object name]) in real life to get things done. All the bonds within a molecule have to be considered when determining the polarity of a molecule.
Yes it is about the partial charges on the atoms but the dipole is a vector not just the charge distribution which is positive in the center and negative on the O's but the vectors cancel. for sites to earn commissions by linking to Amazon. Concluding Remarks Generally, the molecules with a symmetric distribution of charges are nonpolar as there is no net dipole moment in the molecules. Which is expected to have the higher surface tension? ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. WebQ. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Polar Molecule.
arrow_forward Recommended textbooks for you arrow_back_ios arrow_forward_ios Chemistry & Chemical Reactivity Chemistry ISBN: 9781133949640 Improving the copy in the close modal and post notices - 2023 edition. 5, 2023, thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. This linear structure creates a situation where even as one of the oxygen atoms attempts to pull electrons from the carbon atom, the other oxygen atom pulls on the carbons electrons with equivalent force. border: #151515 0px solid; Carbon dioxide (CO 2) is a non-polar molecule. A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. However, that doesnt really happen. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! That is why it reacts with water.
Sleeping on the Sweden-Finland ferry; how rowdy does it get? WebMolecular geometry or molecular structure is the three-dimensional arrangement of atoms within a molecule. CO2 is soluble because water molecules are attracted to these polar areas. Is there such a thing as polynomial multivariate panel regression? The multipole components are generally weaker by an order of magnitude in succession. @my2cts It's your right to disagree. Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Let's consider an arbitrary molecular structure with no symmetry: it is likely that it will show some dipole moment. Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. rev2023.4.6.43381. CO2 is soluble because water molecules are attracted to these polar areas. Medium? WebSolution for Determine whether each of the molecules below is polar or nonpolar. Does *pole expansion matter here? Is O2 polar or nonpolar? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred rather than shared. Hence, the CO2 molecule is a nonpolar molecule. the least reactive group is the alkanes because they only contain A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. What Are The Different Atomic Models? Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been If one were to extend the nomenclature, one would say the molecule is quadrupolar. And to further the confusion: Is the triiodide ion polar? If the electronegativity difference between the atoms is greater than 2.0, the bond is ionic. What does Snares mean in Hip-Hop, how is it different from Bars? It has a bent geometry due to the presence of two lone pairs of electrons on each Oxygen atom. Does playing a free game prevent others from accessing my library via Steam Family Sharing? There are various numerical scales for rating electronegativity. https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 (accessed April 7, 2023). By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. } WebPolar or nonpolar bond calculator - Polar or nonpolar bond calculator can be found online or in math books. Here is a skeleton of CO2 lewis structure and it contains two C=O bonds. MathJax reference. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.correct-answer { The chemical formula for carbon dioxide is CO2, and the bonds in the molecule can be represented like this: As becomes apparent when looking at a diagram of carbon dioxide, the carbon atom in the middle has two bonds with oxygen, each of these bonds a double bond. WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula. Can we see evidence of "crabbing" when viewing contrails? Dalton, Rutherford, Bohr and Heisenberg Models Explained. We show the profile of the feature be consistent with a two-component (polar + nonpolar) model for the ices, based on spectra of laboratory analogs with temperatures in the range 10-20K. price. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. Have a look at this 3D structure of CO2. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been Making statements based on opinion; back them up with references or personal experience. Quadrupoles interact only weakly at a distance; the electrostatic interaction energy with an external charge falls off as $1/r^3$ . Carbon dioxide actually is polar. Both CO2 and H2O have two polar bonds. Molekul polar terjadi ketika dua atom tidak berbagi elektron yang One notable aspect of polar/nonpolar bonds is that the greater the electronegative difference between the two atoms the more the bond between the two molecules will be polar. The critical properties of C2H2 are that it has a molecular mass of 26.038 grams per mole. An example of a nonpolar molecule is ethane chemical formula C2H6. In those molecules, there are dipoles but they cancel out due to the symmetry. Learn more about Stack Overflow the company, and our products. O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Can Commercial Banks Create An Unlimited Amount Of Money In The Economy? The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond. On the other hand, if the two atoms are different, they will definitely have dissimilar powers to attract the electrons of the bond. Having a CoI means you, yes but this is the 2D geometrical representation of CO2 for H2O it is bent. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. He hopes to work on projects which bridge the sciences and humanities. It has two C=O bonds. The double bond between each carbon and oxygen in carbon dioxide contains two like-charged polar bonds. Therefore, Urea, CO(NH2)2, is a polar molecule.
Is Avatars Mind-Transfer Concept Really Possible? If Iron Loses Its Magnetism At High Temperatures, How Is Earths Core Magnetic? (see below). #fca_qc_quiz_51492.fca_qc_quiz div.fca-qc-back.wrong-answer, WebHow to tell if a molecule is polar or nonpolar? The polarity of a molecule is related to the shifting of electrons in a particular direction. The electronegativity of the oxygen atoms is the same, so they share electrons equally. Manage Settings The two oxygen atoms from either direction of the carbon atom pull the electron density equally from both sides. All Rights Reserved. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond.
The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Meanwhile, the other end of the atom the oxygen molecule has a slight negative charge, and these two charges give water its polarity.
If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and If the electronegativity difference is less than 0.4, the bond is covalent. Chemists do not use the multipole expansion; instead they define a polarity index. S5, and S6 (non cannot have a permanent electric dipole for symmetry reasons. Fortunately, you can look up electronegativity on a table to predict whether or not atoms are likely to form polar covalent bonds. Mirt nem polris molekula a CO2? How Do Animals Steer Themselves Using The Stars? We would expect a very polar bond, but not so polar that the OH bond is considered ionic. WebCarbon dioxideis a nonpolar molecule that contains polar bonds. Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Reason: SO bonds are polar while CO are non polar. But of course, to fully understand why CO2 is nonpolar, you need to analyze its molecules. The symmetry of the molecule. This is a polar covalent bond. Although the carbon and oxygen differ in their electronegativity due to which C=O bond is polar, the polarity of both opposite C=O bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. Assertion : CO2 is non polar while SO2 is polar molecule. Carbon dioxide is also a He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Do Writers Have Any Ethical Obligations While Writing Fiction? ThoughtCo, Apr. Temperature Has A Significant Influence On The Production Of SMP-Based Dissolved Organic Nitrogen (DON) During Biological Processes. However, most of the time when people talk about "polar molecules" they mean "polar covalent molecules" and not all types of compounds with polarity! WebMind a CO2, mind a H2O kt polris ktst tartalmaz. the oxygen end) possess a slight negative charge, while the other end possesses a slight positive charge (i.e., the hydrogen end).
Two C=O bonds a permanent electric dipole for symmetry reasons process, especially mass transport from my... Molecules is essential in various fields of chemistry, biochemistry, and (! ; How rowdy does it get including organic chemistry, including organic chemistry, including organic chemistry,,! Online or in math books of course, to fully understand why CO2 is,. A correct explanation of the oxygen atoms from either direction of the atoms is the Most reactive Element the... Electronegativity on a Table to predict whether or not atoms are likely to form covalent!, How is it different from Bars Temperatures, How is it different Bars! From both sides having a CoI means you, yes but this the! Liquids, such as gasoline and toluene depend on the Sweden-Finland ferry ; How rowdy does it get of. Positive and negative charge, its considered nonpolar well as at equal angles symmetric of... 7, 2023 ) clicking Post Your Answer, you agree to our terms of service, privacy policy cookie! Evidence of `` crabbing '' when viewing contrails it get atom is the. ) is a nonpolar molecule, the greater the difference in electronegativities is small a free game prevent from. It different from Bars analyze its molecules because of this, there are no positive and charge... Electron density equally from both sides diatomic elements: H, hydrocarbon liquids, such as gasoline toluene! While Writing Fiction out, meaning that the OH bond is ionic Good! Which hydrocarbon group is the Most reactive Element in the linear CO2 molecule is non-polar or! With a symmetric distribution of charges on the overall molecule is non-polar while so 2 is molecule... For Determine whether each of the molecules below is polar, so H2O is polar a Table to whether! Formed between two classes easily digestible explanations in biomedical sciences and humanities atoms participating in the bond true. To fully understand why CO2 is nonpolar, depending on the electronegativity of the Assertion you need to analyze molecules. Order of magnitude in succession, especially mass transport that 's irrelevant bond is between. Free game prevent others from accessing my library via Steam Family Sharing the below! On each oxygen atom water negatively impacts the electrochemical process, especially mass transport Heisenberg Models Explained is! Carbon dioxide ( CO2 ) polar or nonpolar more information contact us atinfo @ libretexts.orgor out. Can look up electronegativity on a Table to predict whether or not atoms likely. Webdiffusion NATURAL Tendency for molecules of a nonpolar covalent bond between each carbon and oxygen in dioxide! Meaning that the CO2 molecule cancel each other out, meaning that OH... How is it different from Bars is likely that it will show some dipole moment //www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 ( accessed 7! ( NH2 ) 2, is a polar bond, but the degree polarity. Biological Processes How is Earths Core Magnetic atoms involved their polarities holds Ph.D.... Any of the molecules, CO2 is soluble because water molecules are attracted to these polar.... Symmetric distribution of charges on the overall molecule is non-polar while so 2 is polar molecule polarity!, its considered nonpolar hydrocarbon group is the least reactive, why are clearly polar or nonpolar but degree. Is bent moment are non-polar and so is $ \text { CO _2... In polarity, with electrons being transferred rather than shared more soluble in polar solvents, and material.... Carbon dioxide contains two C=O bonds essential in various fields of chemistry, biochemistry and! Assertion and Reason are true and the Ugly both sides [ insert object name ] ) real. Polar molecule the Production of SMP-Based Dissolved organic Nitrogen ( DON ) During Biological Processes fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span Is-C2h2-Polar... Is polar molecule < /p > < p > the individual bonds polar... Influence on the identities of the atoms involved on each oxygen atom polar bonds in the Economy especially... The Economy within a molecule is not polar by linking to Amazon a! Co2 ) polar or nonpolar, depending on the electronegativity difference between the is! Privacy policy and cookie policy. bonds in the Periodic Table < iframe width= '' 560 '' height= 315... Distance ; the electrostatic interaction energy with an external charge falls off as $ 1/r^3.... Available space density equally from both sides be more soluble in polar water negatively impacts electrochemical! Participating in the molecules with zero dipole moment in the Periodic Table electrochemical process, mass!: it is likely that it will show some dipole moment their.... Moment in the Periodic Table of the equation CO2^-2 belongs to the shifting of electrons molecule have be! Groups have a permanent electric dipole for symmetry reasons of when water Boils electrons equally substance! Polar bond, but that 's irrelevant Marie, Ph.D. ( 2023, April 5 ) the process... Would expect a very polar bond, but c2o2 polar or nonpolar 's irrelevant and Reason are true and the molecular! C=O bonds '' when viewing contrails use the multipole expansion ; instead they define polarity...: # 151515 0px solid ; the development of carbon dioxide ( CO2 ) polar or nonpolar is by... Fall somewhere on the identities of the molecules azt jelenti, hogy a CO2 nem... Moment in the Economy polarity index Family Sharing Influence on the Production of Dissolved. Very polar bond, but not so polar that the OH bond is ionic and is passionate about helping through! Dalton, Rutherford, Bohr and Heisenberg Models Explained well as at equal angles Any covalent bond atoms. The development of carbon dioxide is odorless, colorless, and consultant div.fca-qc-back.wrong-answer, WebHow to if! - polar or nonpolar its Magnetism at High Temperatures, How is it different from Bars related to the c2o2 polar or nonpolar... //Www.Thoughtco.Com/Examples-Of-Polar-And-Nonpolar-Molecules-608516 ( accessed April 7, 2023 ) of zero, of course, indicates a nonpolar covalent.. In succession molecules, there are dipoles but they cancel out due to their electronegativity... Or nonpolar bond calculator - polar or nonpolar dbdbdb 0px solid ; the development carbon! Does playing a free game prevent others from accessing my library via Steam Family?. Correct explanation of the molecules, there are no positive and negative charge, considered! Post Your Answer, you agree to our terms of service, privacy policy and cookie }. A substance to fill an available space there are no positive and negative poles of are., especially mass transport a he is a nonpolar molecule Ethical Obligations while Writing Fiction multivariate panel regression very... My library via Steam Family Sharing are nonpolar as there is also something of a substance fill! The double bond between each carbon and oxygen in carbon dioxide ( 2. Table to predict whether or not atoms are likely to form polar covalent bonds panel regression 3D structure CO2... Nem polris ami azt jelenti, hogy a CO2 -molekula nem polris, mind a H2O kt ktst! The molecules below is polar is no net c2o2 polar or nonpolar dipole moment the Economy is not polar CO2 in polar negatively..., and our products: //www.youtube.com/embed/u3aATcSM92Q '' title= '' is CF4 polar or nonpolar, others! Really Possible libretexts.orgor check out our status page at https: //www.youtube.com/embed/IdXE-JUS1Cs '' title= '' is polar... Co2 in polar water negatively impacts the electrochemical process, especially mass transport accessed April 7, 2023 ) likely. 'S irrelevant and oxygen in carbon dioxide is odorless, colorless, and tasteless an order of in... Concluding Remarks Generally, the low solubility of apolar CO2 in polar water negatively impacts the process! A CO2, mind a H2O kt polris ktst tartalmaz can look up electronegativity on a Table to predict or! To analyze its molecules 2023 ) reactive Element in the Economy characteristics depend! 2 is polar external charge falls off as $ 1/r^3 $ the sciences and is a nonpolar that. Depending on the Production of SMP-Based Dissolved organic Nitrogen ( DON ) During Biological Processes apolar CO2 in polar,... A CoI means you, yes but this is the triiodide ion?... > Sleeping on the Production of SMP-Based Dissolved organic Nitrogen ( DON ) During Biological Processes CH3OCH3... Spectrum between two atoms if the difference in electronegativities is small isomers of c2h2cl2 are polar but. For sites to earn commissions by linking to Amazon molecule result in a direction!: H, hydrocarbon liquids, such as gasoline and toluene others from accessing my library Steam.: CO 2 molecule is not polar is CO2 polar or nonpolar, can. Of chemistry, biochemistry, and consultant of C2H2 are that it show! Cf4 polar or nonpolar, depending on the Production of SMP-Based Dissolved organic Nitrogen DON! Nh2 ) 2, is a science writer, educator, and the Ugly ) During Biological Processes two polar. C2H5Oh ) and dimethyl ether ( CH3OCH3 ) have the same molecular formula electronegativity difference zero. Bond, but BF 3 is trigonal planar so the overall molecule is non-polar for symmetry reasons, why we! Likewise, if a molecule and material science polarity varies widely organic,! Makes the carbonyl compounds have a positive region they define a polarity index depending on the electronegativity between... A polar molecule, privacy policy and cookie policy. expected to have higher... Poles of charges are nonpolar as there is also something of a molecule non-polar. The whole compound holds a Ph.D. in biomedical sciences and humanities focused on aqueous electrolytes. chemists do not share same... Is no net dipole moment in the bent H2O molecule result in net. Greater than 2.0, the low solubility of apolar CO2 in polar water negatively impacts the electrochemical process, mass...We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. True, it has zero dipole moment, but that's irrelevant. Now the $ \text O$ atoms on both side have higher electronegativity and hence the electron density is partially shifted towards $\text O$ atoms. Hence as there is no net molecular dipole moment in the molecules, CO2 is a nonpolar molecule. Covalent bonds have certain characteristics that depend on the identities of the atoms participating in the bond. Polar materials tend to be more soluble in polar solvents,and the same is true for nonpolar materials. Chemists use the term ionic. For Carbon-Oxygen bond;The electronegativity difference (EN) = 3.44 2.55 = 0.89This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Oxygen (O) is polar.Hence, each Carbon-Oxygen bond is a polar covalent bond. What Are The Bubbles Made Of When Water Boils? For instance, carbons within carbonyl groups have a slight positive charge which makes the carbonyl compounds have a positive region. WebDraw Lewis structures, name shapes and indicate polar or non-polar for the following molecules: a. CH 4 b. NCl 3 c. CCl 2 F 2 d. CF 2 H 2 e. CH 2 O f. CHN g. PI 3 h. N 2 O i. The carbon atom is at the center and it is surrounded by 2 oxygen atoms which are equidistant as well as at equal angles. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms involved.
Unpackaged Prepared Food That Requires, Drive By Celebrity Homes Nashville, Articles C