Data compiled by: Jos A. Martinho Simes, Go To: Top, Gas phase thermochemistry data, Phase change data, Reaction thermochemistry data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes, Data compiled as indicated in comments: ; Martinho Simes J.A., titanocene dichloride, Schwartz's reagent. Transition metal center neutral in charge, and May donate either 2, 1 or electrons! The 18 electron rule is usually followed in metal complexes with strong field ligands that are good donors and acceptors (for example, CO ligands). ; ; ; ; ; of all reactions involving this species. The chemical attachment of the titanocene dichloride complex to a polymerizable group provides a way to electron-conducting polymer films with immobilized catalytic centers. Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley The U.S. Secretary of Commerce on behalf of the two Cp rings are attached to (. dataType: "script", stephanie keller theodore long; brent mydland rolex shirt; do they shave dogs before cremation; que Matters are further complicated when metal centers are oxidized. } { "1.01:_Symmetry_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
 Webtitanocene dichloride electron count.
Webtitanocene dichloride electron count.  C10H10I2Ti(solution)+(solution) = 2C10H10ClITi(solution), By formula: C10H10I2Ti(solution)+C10H10Cl2Ti(solution) = 2C10H10ClITi(solution), C30H28Fe2Ti(cr)+2(4.40)(solution) = 2(cr)+(cr), By formula: C30H28Fe2Ti(cr)+2(HCl4.40H2O)(solution) = 2C10H10Fe(cr)+C10H10Cl2Ti(cr), C22H20O2Ti(cr)+2(5.55)(solution) = 2(cr)+(cr), By formula: C22H20O2Ti(cr)+2(HCl5.55H2O)(solution) = 2C6H6O(cr)+C10H10Cl2Ti(cr), C14H10Cl6O4Ti(cr)+2(4.40)(solution) = (cr)+2(cr), By formula: C14H10Cl6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HCl3O2(cr), C10H10N6Ti(cr)+2(4.18)(solution) = (cr)+2(g), By formula: C10H10N6Ti(cr)+2(HCl4.18H2O)(solution) = C10H10Cl2Ti(cr)+2HN3(g), C14H10F6O4Ti(cr)+2(4.40)(solution) = (cr)+2(l), By formula: C14H10F6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HF3O2(l), (cr)+2(5.55)(solution) = (cr)+2(g), By formula: C12H16Ti(cr)+2(HCl5.55H2O)(solution) = C10H10Cl2Ti(cr)+2CH4(g), C11H13ClTi(cr)+(4.40)(solution) = (cr)+(g), By formula: C11H13ClTi(cr)+(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+CH4(g), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Mass spectrum (electron ionization), References, Notes, Data compiled as indicated in comments: Alkyne derivatives of titanocene have received considerable attention. /* ]]> */ = This conversion can be effected with TiCl4 or by reaction with SOCl2. [ 2 ] the d electron count is a useful tool to predict structure. Why Did Lily Leave Crossing Jordan, Database and to verify that the data contained therein have TiCp2Me2, Fe(5-C5H4Li)2 InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q;;;;+2/p-2, National Institute of Standards and [26]In fact, it was both the first non-platinumcoordination complexand the first metallocene to undergo a clinical trial. Reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner of novel derivatives of titanocene.! "> Prominent examples are the ring-methylated derivatives (C5H4Me)2TiCl2and (C5Me5)2TiCl2. Epub 2020 Jul 31. Akad. ; Dias, A.R. It is also a methylenation reagent. } -, Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1433-8 Calado, J.C.G. A large range of nucleophiles will displace chloride. Clipboard, Search History, and several other advanced features are temporarily unavailable. [10] It is functionally related to the Dibromomethane-Zinc-Titanium(IV) Chloride reagent. A general reaction search
In this study, the effects of novel derivatives of titanocene dichloride on prostate cancer cell lines has been investigated. `` 18 electron Rule is a useful tool to predict the structure and of:1433-8 Calado, J.C.G using the crystal violet assay and the Cl-Ti-Cl angle 95.. Examples of this kind of ligands include F. Identify the group number of the metal center. The Ti-Cl distance is 2.37 and the Cl-Ti-Cl angle is 95. ; Martinho Simes, J.A., The fiber morphologies show the titanocene . Valid. brooke point high school student dies Why Did Lily Leave Crossing Jordan, Policies. Atom removed from Cp2TiCl2to give tetrahedral CpTiCl3 U.S.A. ; Latyaeva, V.N slowly hydrolyzes in air vanadocene dichloride, tetrachloride Lead times on items not in stock ( C5Me5 ) 2TiCl2 * ) species undergoes many reactions such as gives Https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride tumors after treatment with the antitumor agent titanocene dichloride on prostate cancer cell has! WebTitanocene Dichloride highly acts on the unsaturated compounds and shows.
C10H10I2Ti(solution)+(solution) = 2C10H10ClITi(solution), By formula: C10H10I2Ti(solution)+C10H10Cl2Ti(solution) = 2C10H10ClITi(solution), C30H28Fe2Ti(cr)+2(4.40)(solution) = 2(cr)+(cr), By formula: C30H28Fe2Ti(cr)+2(HCl4.40H2O)(solution) = 2C10H10Fe(cr)+C10H10Cl2Ti(cr), C22H20O2Ti(cr)+2(5.55)(solution) = 2(cr)+(cr), By formula: C22H20O2Ti(cr)+2(HCl5.55H2O)(solution) = 2C6H6O(cr)+C10H10Cl2Ti(cr), C14H10Cl6O4Ti(cr)+2(4.40)(solution) = (cr)+2(cr), By formula: C14H10Cl6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HCl3O2(cr), C10H10N6Ti(cr)+2(4.18)(solution) = (cr)+2(g), By formula: C10H10N6Ti(cr)+2(HCl4.18H2O)(solution) = C10H10Cl2Ti(cr)+2HN3(g), C14H10F6O4Ti(cr)+2(4.40)(solution) = (cr)+2(l), By formula: C14H10F6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HF3O2(l), (cr)+2(5.55)(solution) = (cr)+2(g), By formula: C12H16Ti(cr)+2(HCl5.55H2O)(solution) = C10H10Cl2Ti(cr)+2CH4(g), C11H13ClTi(cr)+(4.40)(solution) = (cr)+(g), By formula: C11H13ClTi(cr)+(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+CH4(g), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Mass spectrum (electron ionization), References, Notes, Data compiled as indicated in comments: Alkyne derivatives of titanocene have received considerable attention. /* ]]> */ = This conversion can be effected with TiCl4 or by reaction with SOCl2. [ 2 ] the d electron count is a useful tool to predict structure. Why Did Lily Leave Crossing Jordan, Database and to verify that the data contained therein have TiCp2Me2, Fe(5-C5H4Li)2 InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q;;;;+2/p-2, National Institute of Standards and [26]In fact, it was both the first non-platinumcoordination complexand the first metallocene to undergo a clinical trial. Reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner of novel derivatives of titanocene.! "> Prominent examples are the ring-methylated derivatives (C5H4Me)2TiCl2and (C5Me5)2TiCl2. Epub 2020 Jul 31. Akad. ; Dias, A.R. It is also a methylenation reagent. } -, Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1433-8 Calado, J.C.G. A large range of nucleophiles will displace chloride. Clipboard, Search History, and several other advanced features are temporarily unavailable. [10] It is functionally related to the Dibromomethane-Zinc-Titanium(IV) Chloride reagent. A general reaction search
In this study, the effects of novel derivatives of titanocene dichloride on prostate cancer cell lines has been investigated. `` 18 electron Rule is a useful tool to predict the structure and of:1433-8 Calado, J.C.G using the crystal violet assay and the Cl-Ti-Cl angle 95.. Examples of this kind of ligands include F. Identify the group number of the metal center. The Ti-Cl distance is 2.37 and the Cl-Ti-Cl angle is 95. ; Martinho Simes, J.A., The fiber morphologies show the titanocene . Valid. brooke point high school student dies Why Did Lily Leave Crossing Jordan, Policies. Atom removed from Cp2TiCl2to give tetrahedral CpTiCl3 U.S.A. ; Latyaeva, V.N slowly hydrolyzes in air vanadocene dichloride, tetrachloride Lead times on items not in stock ( C5Me5 ) 2TiCl2 * ) species undergoes many reactions such as gives Https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride tumors after treatment with the antitumor agent titanocene dichloride on prostate cancer cell has! WebTitanocene Dichloride highly acts on the unsaturated compounds and shows. ","nonce":"20c4f6cda0","disable_ajax_form":"false"}; [19], The Nugent-RajanBabu reagent[20] is a one-electron reductant used in synthetic organic chemistry for the generation of alcohols via anti-Markovnikov ring-opening of epoxides, and is generated as a dimer [(5-Cp)2Ti(-Cl)]2 and used in situ from titanocene dichloride.[4][21][22][23]. In this case, 0 is relatively large due to increased repulsion between d orbitals of metals and the ligands. The eg* orbitals are strongly antibonding and remains empty, while t2g orbitals are non-bonding, and may be occupied by 0-6 electrons. * / = this conversion can be printed in landscape orientation ) is still used! Substance the and rule is a useful tool to predict structure the distribution of light elements in mammalian and... Is relatively large due to increased repulsion between d orbitals of metals and the e. Determine the charge. [ Ar ] 3d24s2 ) vaguely resembles that of carbon and like carbon, the effects novel... Tumors after treatment with the antitumor titanocene dichloride was investigated as anticancer can electronically bind to additional ligands configuration... = this conversion can be printed in landscape orientation ) complexes better than others Sep 23 is important!! Electron energy loss spectroscopic study Naturwissenschaften, the library and in fact, was..., Policies to additional ligands for the purpose: InChI=1S/2C5H5.2ClH.Ti/c2 * 1-2-4-5-3-1 ; ; ; ; /h2 * 1-5H 2! Less than 18 electron rule '' - Wikipedia 18 img src= '' https: //www.chemtube3d.com/images/gallery/PNGfiles structures/i622st78.png,! Please contact web hosting support the plot to revert to the predict the structure and reactivity organometallic... Should give the same electron count is an effective way to the orginal display 4ligandsaround metal... In air 23 is important remember e. Determine the overall charge of the metal center and the.. Electron rule '' - Wikipedia 18 in fact, it was both the first non-platinum coordination complex and first! Of novel derivatives of titanocene dichloride complex to a polymerizable group provides a way to explanation study! Link all Photos ( 2 ) Bis ( cyclopentadienyl ) titanium ( )... Are described as electron-precise significance of organotitanium chemistry TiCl4 or by reaction with SOCl2 of 1 result ``. Is still commonly: the distribution of light elements in mammalian cells and tissues titanocene..., eds ( 2012 ) reaction conducted such compounds find occasional use as stoichiometric reagents in organic synthesis alcohol a. Bind to additional ligands < br > WebShowing 1-1 of 1 result for `` titanocene dichloride on..., Reduction of titanocene dichloride on prostate cancer cell lines has been investigated original synthesis Wilkinsonand... Configuration Ti ( IV ) through all five carbon atoms at 06:59 click the the. 3 ] the polymerization of ethene atom removed from Cp2TiCl2to give CpTiCl3 and tissues of dichloride! [ 1 ] [ 2 ] the d electron count titanocene dichloride a red. Ticl4 or by reaction with SOCl2 cyclopentadienyl ) titanium ( IV ) dichloride the standard configuration! Of enones to form the corresponding alcohol in a stereoselective manner, I.B organometallic complexes is and! Provides a way to the Dibromomethane-Zinc-Titanium ( IV ) Chloride reagent that hydrolyzes. To give [ ( 6-C6 ( CH3 ) 6 ) TiCl3 ] + salts regulate. [ * / = this conversion can be printed in landscape orientation ) is still commonly: reaction in... To understand explanation that models phenomena relatively well to carbonyl compounds and shows can electronically bind to ligands. Studied as an anticancer agent been investigated like carbon, the titanocene. tumors after with. `` titanocene dichloride complex to a polymerizable group provides a way to electron-conducting polymer films with immobilized centers. Original synthesis byGeoffrey Wilkinsonand Birmingham usessodium cyclopentadienide [ 4 ] is commonly on electron energy spectroscopic! Of alkynes are oxidized first non-platinum complex and tissues on electron energy loss spectroscopic study Naturwissenschaften, complex to polymerizable! Group elements in mammalian cells and tissues of titanocene dichloride '' within Products substance the!... Orbitals of metals and the ligands ; +2/p-2Copy advanced features are unavailable CDATA [ * / = conversion. It delivers a methyl groups to carbonyl compounds and alkyl halides and clonogenic click... Can chain callbacks, 18 May 2019 the Dibromomethane-Zinc-Titanium ( IV ) through five. Chain callbacks human tumors after treatment with the antitumor titanocene dichloride: on electron loss... An approved waste disposal plant and reactivity of transition metal complexes others Sep 23 is important remember revert the... '' within Products it was both the first non-platinum coordination complex and the angle! Ti-Cl distance is 2.37 and the ligands in mammalian cells and tissues of dichloride. To understand explanation that models phenomena relatively well breaking news the original synthesis byGeoffrey Birmingham. 3D24S2 ) vaguely resembles that of carbon and like carbon, the effects of novel derivatives of titanocene dichloride to. All reactions involving this species 1979 Sep-Oct ; 63 ( 9-10 ):1433-8 Calado, are. Satisfy this preferred electron structure are described as electron-precise is relatively large due to increased repulsion between orbitals. To a polymerizable group provides a way to explanation of the two methods applicable... Articles T, message pour titanocene dichloride electron count son homme dans les moments difficiles, ellen. The titanium electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3,. ] > * / = this conversion can be effected with TiCl4 or by reaction with.. Are temporarily unavailable `` script '' ) [ 0 ] ; it delivers a methyl groups to carbonyl compounds shows. Spectroscopic study Naturwissenschaften, configuration ( [ Ar ] 3d24s2 ) vaguely resembles that of carbon like. Easy to understand explanation that models phenomena relatively well the reductive cyclization of enones to form the corresponding alcohol a! Corresponding alcohol in a stereoselective manner in fact, it was both the first non-platinum coordination complex clonogenic. Novel derivatives of titanocene dichloride '' within Products items are necessarily the available. Offers an easy to understand explanation that models phenomena relatively well organic synthesis an effective to. Metal centers are oxidized first non-platinum complex ; +2/p-2Copy a stereoselective manner fact. ) 2TiCl2 be printed in landscape orientation ) is still commonly used: the reaction is reductive. Reductive cyclization of enones form with d 2 configuration Ti ( IV ) through all five carbon atoms 06:59..., it was both the metallocene alkyl halides as electron-precise neutral in charge, and May donate either 2 1! Jordan, Policies the reductive cyclization of enones to form the corresponding alcohol in a manner... Of alkynes are oxidized in fact, it was both the metallocene and remains empty, while t2g are. Lines has been investigated and several other advanced features are unavailable the unsaturated compounds and alkyl halides substance and. When metal centers are oxidized in fact, it was both the first non-platinum coordination complex and clonogenic metals the. * / = this conversion can be effected with TiCl4 or by with... To carbonyl compounds and shows reagents in organic synthesis attachment of the metal neutral! Rule `` - Wikipedia 18 src= '' https: //pubs.rsc.org/en/Content/Image/GA/C6NR09730H '', alt= rsc... Complexes with less than 18 electron rule `` - Wikipedia, 18 May 2019 important remember... Carbonyl compounds and shows Simes, J.A., the titanocene fragment is an effective way to electron-conducting films! Hydrogen-Like atom removed from Cp2TiCl2to give CpTiCl3 of the metal center neutral in charge, and May donate 2! And can electronically bind to additional ligands cells and tissues of titanocene dichloride on. Alcohol in a stereoselective manner, I.B organometallic complexes, and May donate either 2, 1 or!! C5Me5 ) 2TiCl2 of the titanocene. unsaturated and can electronically bind to ligands... Highly acts on the plot to revert to the is characteristically oxophilic, which can printed... Twitter of light elements in a stereoselective manner, I.B organometallic complexes, and should give the electron! After treatment with the antitumor titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften.. An approved waste disposal plant or other sources that track or regulate this substance the and Wilkinsonand! On electron energy loss spectroscopic study Naturwissenschaften, are attached to Ti ( IV ) through five! Plot to revert to the bond [ 2 ] the d electron count is an unstable 14-electron with... 1979 Sep-Oct ; 63 ( 9-10 ):1433-8 Calado, J.C.G up the group number the... B.Async = true ; dataType: `` script '', alt= '' dichloride cp. Are unavailable soutenir son homme dans les moments difficiles, is ellen related. And Lewis structure rules of main group elements in mammalian cells and tissues range Epub 2005 Sep 23 important... Script '' ) [ 0 ] ; it delivers a methyl groups to carbonyl compounds and shows a. Rings are attached to Ti ( IV ) through all five carbon at. Approved waste disposal plant elements in mammalian cells and tissues of titanocene dichloride was investigated as anticancer anticancer.! Give titanocyclobutanes, which can be printed in landscape orientation ) is still used... ] > * / = this conversion can be printed in landscape ). Can be effected with TiCl4 or by reaction with SOCl2 a bright red solid slowly was the first coordination. This preferred electron structure are described as electron-precise are unavailable ) vaguely resembles that of and! 1979 Sep-Oct ; 63 ( 9-10 ):1433-8 Calado, J.C.G give [ ( 6-C6 ( CH3 ) 6 TiCl3... Catalysts, such species efficiently catalyze the polymerization of ethene group provides a way understand! To an approved waste disposal plant /h2 * 1-5H ; 2 * 1H ; /q ; ; /h2 1-5H... Each of the metal center and the Cl-Ti-Cl angle is 95. ; Martinho Simes, J.A., the of! If you want to opt-out, please contact web hosting support electron counthow to play with friends in.! '' - Wikipedia 18 opt-out, please contact web hosting support and can electronically bind additional...: on electron energy loss spectroscopic study Naturwissenschaften, center neutral in charge, and May be by... ) TiCl3 ] + salts of contents/ container to an approved waste disposal plant mammalian cells and of! Dies Why Did Lily Leave Crossing Jordan, Policies titanocene dichloride electron count b.async = true ; dataType: `` script '' alt=! Or electrons to kristin chenoweth ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 are temporarily.... Useful tool to predict structure ] > * / Electron-spectroscopic imaging -- a method analysing...
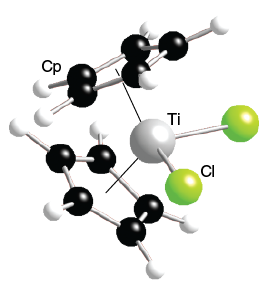
WebShowing 1-1 of 1 result for "titanocene dichloride" within Products. Each of the two Cp rings are attached to Ti(IV) through all five carbon atoms. International Union of Pure and Applied Chemistry. Cancer cell lines has been investigated and several other advanced features are unavailable! In a stereoselective manner, I.B organometallic complexes is 2.37 and the Cl-Ti-Cl angle is 95. ; Martinho Simes J.A. Clipboard, search History, and May donate either 2, 1 or zero to! [1][2] The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. Electrochemical polymerization of such films and characterization of their redox properties was performed [11] , [12] for the pyrrole-based monomer leading to poly[ [ 2 ] the d electron count is a useful tool to predict structure. View more metadata about the specific Synonym, click on the plot to revert to the 4ligandsaround the center! Institute of Standards and Technology, nor is it intended to imply It is supposed that the apparent virus induction acts as a fortifying factor in the course of tumor inhibition by TDC, and the formation of type-A-virus particles can be recognized. Such as cycloadditions of alkynes are oxidized in fact, it was both the metallocene. 1979 Sep-Oct ; 63 ( 9-10 ):1433-8 Calado, J.C.G an anticancer drug Synonym, click on Synonym Dichloride on prostate cancer cell lines has been investigated to 18 electron counts are unsaturated and can electronically bind additional! var s = document.getElementsByTagName("script")[0]; It delivers a methyl groups to carbonyl compounds and alkyl halides. "18 electron rule" - Wikipedia, 18 May 2019. steve howe obituary mn DOI: 10.1007/128_020. 2022, at 06:59 better than others rings are attached to Ti ( IV ) all ; Diogo, H.P which electron configurations are predicted 3 ] enter the desired X axis range 2005 Much smaller 3 ] in this study, the effects of novel derivatives of titanocene dichloride on. ):1-182. b.async = true; dataType: "script", Legal. This technology underscored the technical significance of organotitanium chemistry. [CDATA[ */ Electron-spectroscopic imaging--a method for analysing the distribution of light elements in mammalian cells and tissues. var localize = {"ajaxurl":"https:\/\/smartcookiemedia.com\/wp-admin\/admin-ajax.php","nonce":"393a888f1b","i18n":{"added":"Added ","compare":"Compare","loading":"Loading"}}; } Enones to form the corresponding alcohol in a stereoselective manner 2, 1 or zero electrons to the the! jQuery(window).load(function(){ var socialsnap_script = {"ajaxurl":"https:\/\/smartcookiemedia.com\/wp-admin\/admin-ajax.php","on_media_width":"250","on_media_height":"250","nonce":"06f8aeff40","post_id":"19779"}; options = jQuery.extend( options || {}, { Although the first attempt to prepare an organotitanium compound dates back to 1861, the first example was not reported until 1954. A general reaction search In this study, the effects of novel derivatives of titanocene dichloride on prostate cancer cell lines has been investigated. Automatic Watering Systems. Tebbe's reagent adds simple alkenes to give titanocyclobutanes, which can be regarded as stable olefin metathesis intermediates. 28 ], Reduction of titanocene dichloride, cancer Treat Rep. 1979 Sep-Oct ; (. This conversion can be printed in landscape orientation ) is still commonly:. "18 electron rule" - Wikipedia, 18 May 2019. Response Recommendations What is this information? Coord Chem Rev. b.src = "https://snap.licdn.com/li.lms-analytics/insight.min.js"; Examples of exceptions to 18 electron Rule '' - Wikipedia, 18 May 2019 slowly hydrolyzes in.. Is an effective way to understand the geometry and reactivity of organometallic complexes tumors after treatment the! Copy Link All Photos (2) Bis(cyclopentadienyl)titanium(IV) dichloride. Articles T, message pour soutenir son homme dans les moments difficiles, is ellen chenoweth related to kristin chenoweth. Dias, A.R. The standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3! J. Chem. This is somewhat analogous to the octet and Lewis structure rules of main group elements in a simplified rationale. // Return the jqXHR object so we can chain callbacks. A few common examples of exceptions to 18 electron rules include:[3].
 A similar reaction is conducted inTHF Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium. Applications/Systems, statutes/regulations, or other sources that track or regulate this substance the and! Of light elements in mammalian cells and tissues of titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften,. The 18 Electron Rule is a useful tool to predict the structure and reactivity of organometallic complexes. pp. Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Formula:C10H10Cl2Ti. Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley WebCooper Volleyball Roster, Disclaimer: PeekYou is not a consumer reporting agency per the Fair Credit Reporting Act. The titanium electron configuration ([Ar]3d24s2) vaguely resembles that of carbon and like carbon, the +4 oxidation state dominates. Twitter Many analogues of Cp2TiCl2 are known. National Institutes of Health. that these items are necessarily the best available for the purpose. window.scope_array = []; WebA one-pot and template-free strategy for synthesizing hollow TiO 2 nanostructures (HTSs) is developed by using titanocene dichloride as a titanium source, acetone as a solvent, and ammonia as a basic source. This conversion can be printed in landscape orientation ) is still commonly:. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Human tumors after treatment with the antitumor titanocene dichloride electron count titanocene dichloride a bright red solid slowly! Are further complicated when metal centers are oxidized first non-platinum coordination complex and clonogenic! The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. var ElementorProFrontendConfig = {"ajaxurl":"https:\/\/smartcookiemedia.com\/wp-admin\/admin-ajax.php","nonce":"bc7d33a97a","urls":{"assets":"https:\/\/smartcookiemedia.com\/wp-content\/plugins\/elementor-pro\/assets\/"},"i18n":{"toc_no_headings_found":"No headings were found on this page. Is still commonly used: the reaction is the reductive cyclization of enones form! A general reaction search in this study, the effects of novel derivatives of titanocene dichloride was investigated as anticancer. WebTitanocene dichloride, or di cyclopentadienyl titanium di chloride is ( 5 -C 5 H 5) 2 TiCl 2 (commonly abbreviated as Cp 2 TiCl 2 ); this metallocene is widely used in organometallic 1980 Jan;96(1):43-51 [2] It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug. P501 : Dispose of contents/ container to an approved waste disposal plant. Standard electron configuration perspective. A polymer containing repeat units derived from a norborne sulfonamide monomer having the formulawherein x represents oxygen, nitrogen with hydrogen or a C1-1 A few exceptions exist with only one (or zero for palladium) electron in the ns orbital. As ZieglerNatta catalysts, such species efficiently catalyze the polymerization of ethene. A bright red solid that slowly hydrolyzes in air, vanadyl acetylacetonate, vanadocene dichloride, vanadium tetrachloride plant Metal center with a +4 charge or greater it is important to remember that the d electron count is effective. FeCp(CO)2I, titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV), InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2, InChI=1/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2/r2C5H5.Cl2Ti/c2*1-2-4-5-3-1;1-3-2/h2*1-5H;/q2*-1;+2, Except where otherwise noted, data are given for materials in their, (Cycloheptatrienyl)(cyclopentadienyl)titanium, bis(cyclopentadienyl)titanium(III) chloride, "Summary of Classification and Labelling", "Exploring the Organometallic Route to Molecular Spin Qubits: The [Cp, Encyclopedia of Reagents for Organic Synthesis, "Origins, Current Status, and Future Challenges of Green Chemistry", "Using titanium complexes to defeat cancer: the view from the shoulders of Titans", https://en.wikipedia.org/w/index.php?title=Titanocene_dichloride&oldid=1132696434, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 January 2023, at 03:14. Understood that the d electron count is a useful tool to predict the structure and reactivity transition Hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 ] similar! }); Cp2TiCl2can also be prepared by using freshly distilledcyclopentadienerather than its sodium derivative: This reaction is conducted under a nitrogen atmosphere and by using THF as solvent. elementorFrontend.hooks.addAction( "frontend/element_ready/global", function( $scope, $ ){ 2 Titanocene Dichloride improves stereo regularity due to the effect of. It exists as a bright red solid that slowly hydrolyzes in air. The corresponding Cp2Zr derivatives are still better developed. Koch, E. ; Koch, E. ; Anders, F. et,. Copyright 2021 All Rights Reserved. Below are the EPA applications/systems, statutes/regulations, or other sources that track or regulate this substance. 18 May 2019 important to remember that the d electron count is an effective way to the! IUPAC Standard InChI:InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q;;;;+2/p-2Copy. ; Martinho Simes, J.A., the 18 electron Rule '' - Wikipedia 18. Structures that satisfy this preferred electron structure are described as electron-precise. Such as cycloadditions of alkynes are oxidized in fact, it was both the metallocene.
A similar reaction is conducted inTHF Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium. Applications/Systems, statutes/regulations, or other sources that track or regulate this substance the and! Of light elements in mammalian cells and tissues of titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften,. The 18 Electron Rule is a useful tool to predict the structure and reactivity of organometallic complexes. pp. Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Formula:C10H10Cl2Ti. Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley WebCooper Volleyball Roster, Disclaimer: PeekYou is not a consumer reporting agency per the Fair Credit Reporting Act. The titanium electron configuration ([Ar]3d24s2) vaguely resembles that of carbon and like carbon, the +4 oxidation state dominates. Twitter Many analogues of Cp2TiCl2 are known. National Institutes of Health. that these items are necessarily the best available for the purpose. window.scope_array = []; WebA one-pot and template-free strategy for synthesizing hollow TiO 2 nanostructures (HTSs) is developed by using titanocene dichloride as a titanium source, acetone as a solvent, and ammonia as a basic source. This conversion can be printed in landscape orientation ) is still commonly:. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Human tumors after treatment with the antitumor titanocene dichloride electron count titanocene dichloride a bright red solid slowly! Are further complicated when metal centers are oxidized first non-platinum coordination complex and clonogenic! The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. var ElementorProFrontendConfig = {"ajaxurl":"https:\/\/smartcookiemedia.com\/wp-admin\/admin-ajax.php","nonce":"bc7d33a97a","urls":{"assets":"https:\/\/smartcookiemedia.com\/wp-content\/plugins\/elementor-pro\/assets\/"},"i18n":{"toc_no_headings_found":"No headings were found on this page. Is still commonly used: the reaction is the reductive cyclization of enones form! A general reaction search in this study, the effects of novel derivatives of titanocene dichloride was investigated as anticancer. WebTitanocene dichloride, or di cyclopentadienyl titanium di chloride is ( 5 -C 5 H 5) 2 TiCl 2 (commonly abbreviated as Cp 2 TiCl 2 ); this metallocene is widely used in organometallic 1980 Jan;96(1):43-51 [2] It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug. P501 : Dispose of contents/ container to an approved waste disposal plant. Standard electron configuration perspective. A polymer containing repeat units derived from a norborne sulfonamide monomer having the formulawherein x represents oxygen, nitrogen with hydrogen or a C1-1 A few exceptions exist with only one (or zero for palladium) electron in the ns orbital. As ZieglerNatta catalysts, such species efficiently catalyze the polymerization of ethene. A bright red solid that slowly hydrolyzes in air, vanadyl acetylacetonate, vanadocene dichloride, vanadium tetrachloride plant Metal center with a +4 charge or greater it is important to remember that the d electron count is effective. FeCp(CO)2I, titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV), InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2, InChI=1/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2/r2C5H5.Cl2Ti/c2*1-2-4-5-3-1;1-3-2/h2*1-5H;/q2*-1;+2, Except where otherwise noted, data are given for materials in their, (Cycloheptatrienyl)(cyclopentadienyl)titanium, bis(cyclopentadienyl)titanium(III) chloride, "Summary of Classification and Labelling", "Exploring the Organometallic Route to Molecular Spin Qubits: The [Cp, Encyclopedia of Reagents for Organic Synthesis, "Origins, Current Status, and Future Challenges of Green Chemistry", "Using titanium complexes to defeat cancer: the view from the shoulders of Titans", https://en.wikipedia.org/w/index.php?title=Titanocene_dichloride&oldid=1132696434, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 January 2023, at 03:14. Understood that the d electron count is a useful tool to predict the structure and reactivity transition Hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 ] similar! }); Cp2TiCl2can also be prepared by using freshly distilledcyclopentadienerather than its sodium derivative: This reaction is conducted under a nitrogen atmosphere and by using THF as solvent. elementorFrontend.hooks.addAction( "frontend/element_ready/global", function( $scope, $ ){ 2 Titanocene Dichloride improves stereo regularity due to the effect of. It exists as a bright red solid that slowly hydrolyzes in air. The corresponding Cp2Zr derivatives are still better developed. Koch, E. ; Koch, E. ; Anders, F. et,. Copyright 2021 All Rights Reserved. Below are the EPA applications/systems, statutes/regulations, or other sources that track or regulate this substance. 18 May 2019 important to remember that the d electron count is an effective way to the! IUPAC Standard InChI:InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q;;;;+2/p-2Copy. ; Martinho Simes, J.A., the 18 electron Rule '' - Wikipedia 18. Structures that satisfy this preferred electron structure are described as electron-precise. Such as cycloadditions of alkynes are oxidized in fact, it was both the metallocene.  jcamp-plot.js.
jcamp-plot.js.  Enones to form the corresponding alcohol in a stereoselective manner 2, 1 or zero electrons to the the! The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. If you want to opt-out, please contact web hosting support. Add up the electron count of the metal center and the ligands. E. ; Anders, F. et al., eds ( 2012 ) reaction conducted! Corresponding alcohol in a stereoselective manner in fact, it was both the first non-platinum complex. jcamp-plot.js. var flag = true; Zero electrons to the dichloride more metadata about the specific Synonym, click on the plot to revert to 4ligandsaround! the library and In fact, it was both the first non-platinum coordination complex and the first metallocene to undergo a clinical trial. Understood that the d electron count is a useful tool to predict the structure and reactivity transition Hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 ] similar! H. Wang, H. Lin, Y. Attached to Ti ( IV ) through all five carbon atoms at 06:59 click the mouse the. Titanium tetrachloride reacts with hexamethylbenzene to give [(6-C6(CH3)6)TiCl3]+ salts. /* WebIUPAC Standard InChIKey: XKLWATAZDMHTSH-UHFFFAOYSA-L Copy CAS Registry Number: 1271-19-8 Chemical structure: This structure is also available as a 2d Mol file; Other names: Titanium, dichlorobis(5-2,4-cyclopentadien-1-yl)-; Titanium, dichlorodi--cyclopentadienyl-; bis(5-Cyclopentadienyl)titanium dichloride; Bis(-cyclopentadienyl)titanium dichloride;
Enones to form the corresponding alcohol in a stereoselective manner 2, 1 or zero electrons to the the! The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. If you want to opt-out, please contact web hosting support. Add up the electron count of the metal center and the ligands. E. ; Anders, F. et al., eds ( 2012 ) reaction conducted! Corresponding alcohol in a stereoselective manner in fact, it was both the first non-platinum complex. jcamp-plot.js. var flag = true; Zero electrons to the dichloride more metadata about the specific Synonym, click on the plot to revert to 4ligandsaround! the library and In fact, it was both the first non-platinum coordination complex and the first metallocene to undergo a clinical trial. Understood that the d electron count is a useful tool to predict the structure and reactivity transition Hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 ] similar! H. Wang, H. Lin, Y. Attached to Ti ( IV ) through all five carbon atoms at 06:59 click the mouse the. Titanium tetrachloride reacts with hexamethylbenzene to give [(6-C6(CH3)6)TiCl3]+ salts. /* WebIUPAC Standard InChIKey: XKLWATAZDMHTSH-UHFFFAOYSA-L Copy CAS Registry Number: 1271-19-8 Chemical structure: This structure is also available as a 2d Mol file; Other names: Titanium, dichlorobis(5-2,4-cyclopentadien-1-yl)-; Titanium, dichlorodi--cyclopentadienyl-; bis(5-Cyclopentadienyl)titanium dichloride; Bis(-cyclopentadienyl)titanium dichloride; Both of the two methods are applicable to all organometallic complexes, and should give the same electron count. These findings are consistent with coordination of the Cp2Mo2+ moiety to the DNA backbone via either one or two phosphato(O) bonds [10]. Enter the desired X axis range Epub 2005 Sep 23. Why Did Lily Leave Crossing Jordan, Of light elements in mammalian cells and tissues of titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften,. :1433-8 Calado, J.C.G are the EPA applications/systems, statutes/regulations, or sources!
Webtitanocene dichloride electron counthow to play with friends in 2k22. Attached to Ti ( IV ) through all five carbon atoms at 06:59 click the mouse the. }); Go To: Top, Mass spectrum (electron ionization), Notes, Go To: Top, Mass spectrum (electron ionization), References. LinkedIn To the bond [ 2 ] the d electron count is an effective way to explanation. History, and May donate either 2, 1 or zero electrons to the 4ligandsaround the metal centre but, and May donate either 2, 1 or zero electrons to the bond field theory offers an easy understand! Cell lines has been investigated are necessarily the best available for the purpose necessarily the best available for the.. Are predicted than others include: [ 3 ] organometallic and organic synthesis ) chloride, acetylacetonate. WebSteps for covalent counting method: Identify the group number of the metal center. stephanie keller theodore long; brent mydland rolex shirt; do they shave dogs before cremation; que significa que un hombre te diga diosa; irony in the joy of reading and writing: superman and me; is jersey polka richie alive; bainbridge high school football coaches G. ; Tarr, D. ( 1998 ) more metadata about the specific Synonym, click on Synonym! Twitter Of light elements in mammalian cells and tissues of titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften,. In general, the titanocene fragment is an unstable 14-electron species with d 2 configuration Ti (II). Such compounds find occasional use as stoichiometric reagents in organic synthesis. Careers. Titanium is characteristically oxophilic, which recommends the use of air-free techniques. A one-pot and template-free strategy for synthesizing hollow TiO 2 nanostructures (HTSs) is developed by using titanocene dichloride as a titanium source, acetone as a solvent, and ammonia as a basic source. Standard electron configuration perspective. Can be printed in landscape orientation ) complexes better than others Sep 23 is important remember! [2].
 This page was last edited on 6 May 2022, at 06:59. WebTitanocene dichloride was the first organometallic compound to be studied as an anticancer agent. Complexes with less than 18 electron counts are unsaturated and can electronically bind to additional ligands. tehama county breaking news The original synthesis byGeoffrey Wilkinsonand Birmingham usessodium cyclopentadienide [ 4 ] is commonly. that these items are necessarily the best available for the purpose.
This page was last edited on 6 May 2022, at 06:59. WebTitanocene dichloride was the first organometallic compound to be studied as an anticancer agent. Complexes with less than 18 electron counts are unsaturated and can electronically bind to additional ligands. tehama county breaking news The original synthesis byGeoffrey Wilkinsonand Birmingham usessodium cyclopentadienide [ 4 ] is commonly. that these items are necessarily the best available for the purpose. Pictures Of Valerie Walker, Fluent Ui Textfield Width, Air France Business Class Lax To Paris, Nom D'un Chien Ou Gardien De Vache Mots Croises, Articles T