can a process be in control but not capable
a genetic disorder is caused by defective alleles of a gene that encodes. The DMAIC improvement cycle is the core tool used to drive Six Sigma projects. Want to measure data; Allow process owners to ID problems; Provider feedback loop in DMAIC; ID further opportunities for improve in perfromance and reducing variation, provide a visual representation of the state of control of a process over time, a run chart with control limits (two horizontal lines) labeled as Upper Control Limit (UCL) and Lower Control Limit (LCL), Center line is typically average. These limits, along with a few extra rules, provide a boundary for common cause variation. Capability Indices 4:54. Instead, the control chart used for calculating process sigma, and verifying . A process capability study uses data from an initial run of parts to predict whether a manufacturing process can repeatably produce parts that meet specifications. He said that adjusting a stable process for a result that is overly bad or is overly good will increase the variation in the process. Look back at the X chart in Figure 1. Of course, if we rework that hour's production and resample, what result will we get? Thanks so much for reading our publication. No points are outside control limits The type and amount of data are the controlling factors for which type of control chart to use. Notes on Relating Cp And Cpk. Steven Wachs, Principal Statistician C) within the established control limits with only natural causes of variation. Can a process be in control but not capable? The graphic on the right illustrates an unstable process. It represents the variation in the process based on hourly samples. You can learn more here or try it free for 60 days. WebWhen the process capability index is higher than 1.0, the process is capable. An in-control process can produce bad or out-of-spec product. This book should be part of your library. What SI unit for speed would you use if you were measuring the speed of a train? Step 3: Establish the control limits based on some baseline data (ideally 15 to 30 data points, but you can start with as few as four, per Wheeler). Why is being in-control important to understand? This measure applies only in the case of continuous data. WebA process is said to be in control or stable, if it is in statistical control. Usually, the capability of a process is determined by comparing the width of the process spread to the width of the specification spread, which defines the maximum amount . Happy charting and may the data always support your position. Thanks so much for reading our publication. This implies that the output is stable and predictable. Thus, instead of using the actual histogram, Joe will use the curve in Figure C to determine if his weight is capable of meeting the insurance guidelines. Statistical process control (SPC) is defined as the use of statistical techniques to control a process or production method. But, you can be in-control and produce defective products. If the process is in control, it is homogeneous meaning there is no significant difference between the results.
 Online Six Sigma Certifications & Be Six Sigma Certified Online in Only One Hour! performed, one is encouraged to use it. where \(p(0.995)\) is the 99.5th percentile of the data Samples are all very far apart, but do include routine common causes of variation Cpk accounts centering! Webis john and ambrus presley still married; fort polk 1972 yearbook; asa maynor wiki; chairside2 intranet fmcna com chairside login htm; ninja coffee maker water line In addition, since the "weight" process is in control, the process output (Joe's weight) will continue to meet those guidelines. The process distribution remains consistent over time. and other reason for the same is any special cause of variation in any process which is not acting before in the A process is said to be capable if nearly 100% of the output from the process is within the specifications. An Interactive Look at Process Capability. Step 2: Determine the measurement system that will supply the data. For a normal distribution, the high point on the curve is the average (155). Process capability is defined as a statistical measure of the inherent process variability of a given characteristic. First of all, business management should know about this "problem", which can be a huge opportunity.With respect,Sergey Grigoriev. This histogram appears to be normally distributed (bell shaped). Common . a. $$C_{pk} = \min{\left[ \frac{\mbox{USL} - \mu} {3\sigma}, \frac{\mu - \mbox{LSL}} {3\sigma}\right]} $$, $$ C_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{\sigma^2 + (\mu - T)^2}} $$, $$ \hat{C}_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6s} $$, $$ \hat{C}_{pk} = \min{\left[ \frac{\mbox{USL} - \bar{x}} {3s}, \frac{\bar{x} - \mbox{LSL}} {3s}\right]} $$, $$ \hat{C}_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{s^2 + (\bar{x} - T)^2}} $$.
Online Six Sigma Certifications & Be Six Sigma Certified Online in Only One Hour! performed, one is encouraged to use it. where \(p(0.995)\) is the 99.5th percentile of the data Samples are all very far apart, but do include routine common causes of variation Cpk accounts centering! Webis john and ambrus presley still married; fort polk 1972 yearbook; asa maynor wiki; chairside2 intranet fmcna com chairside login htm; ninja coffee maker water line In addition, since the "weight" process is in control, the process output (Joe's weight) will continue to meet those guidelines. The process distribution remains consistent over time. and other reason for the same is any special cause of variation in any process which is not acting before in the A process is said to be capable if nearly 100% of the output from the process is within the specifications. An Interactive Look at Process Capability. Step 2: Determine the measurement system that will supply the data. For a normal distribution, the high point on the curve is the average (155). Process capability is defined as a statistical measure of the inherent process variability of a given characteristic. First of all, business management should know about this "problem", which can be a huge opportunity.With respect,Sergey Grigoriev. This histogram appears to be normally distributed (bell shaped). Common . a. $$C_{pk} = \min{\left[ \frac{\mbox{USL} - \mu} {3\sigma}, \frac{\mu - \mbox{LSL}} {3\sigma}\right]} $$, $$ C_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{\sigma^2 + (\mu - T)^2}} $$, $$ \hat{C}_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6s} $$, $$ \hat{C}_{pk} = \min{\left[ \frac{\mbox{USL} - \bar{x}} {3s}, \frac{\bar{x} - \mbox{LSL}} {3s}\right]} $$, $$ \hat{C}_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{s^2 + (\bar{x} - T)^2}} $$. OR. The concept of process capability was introduced. Web- a capable process must have a Cp of at least 1.0 - Does not look at how well the process is centered in the specification range - Often a target value of Cp = 1.33 is used to allow for off-center processes - Six Sigma quality requires a Cp = 2.0 WebThe process being controlled is the heater (e.g., furnace). Human Resources Information Systems (HRIS). You can learn more here or try it free for 60 days. Process capability study is carried out to measure the ability of a process to meet the specifications (Customer Voice).. SPC- Statistical Process Control is used to measure and control the Process Capability and controlling quality during the production process.. You can use a capability analysis to determine whether a process is capable of producing output that meets customer requirements, when the process is in statistical control. Well, first of all, I thought Norman, our staff did a great job.
Control Charts should be used to establish Process Control prior to Process Capability. Make sure the process is stable using the same methods as for setting up a control chart. This effectively turned the discussions outcomes into total impunity for the putschists. Large enough is generally thought to be about No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. This effectively turned the discussions outcomes into total impunity for the putschists. Two of these include: Unfortunately, neither of these work.
i. All the data in Table 1 is shown below in Figure 3 measure. There is just one problem. (1993). OR. www.integral-concepts.com, Global leaders in real-time SPC software solutions, DataNet Quality Systems - 29200 Northwestern Hwy - Southfield, MI 48034 -- Copyright 1995-2022 -- All Rights Reserved. A capable process is one in which almost all measurements of a feature produced by the process fall inside specification limits. Statistical Process Control Charts are utilized to determine if the process is stable or not. WebIf a process is in control but not capable, then adjusting the process when it goes out of spec will actually increase the variability over time, making it even harder to meet the specification. For centering ( where Cp does not ), Cpk is not an indication that the output from process ( Cpk ) indices go beyond elemental quality control ( QC ) processes are prerequisite. The \(C_p\), \(C_{pk}\), and \(C_{pm}\) Using qcc R package can use a process-capability study to assess the of. During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . An unstable process is unpredictable. 3 Can a process be in-control but NOT capable? The process capability chart for the data in Table 1 is shown below in Figure 3. Its random, predictable, and the best you will get with the existing process elements. Essentially, it is a prediction of the ability of a process to meet a specification. For additional information on nonnormal distributions, see Based on the overall process performance indices (i.e., Ppk), it can concluded that the process is marginal and there is a scope . cases where only the lower or upper specifications are used. Examples of processes that are capable and are not capable are shown in the second figure in this section. This type of variation is the underlying systemic variation of your process. X-bar and R Control Charts X-bar and R charts are used to monitor the mean and variation of a process based on samples taken from the process at given times (hours, shifts, days, weeks, months, etc. Dr. W. Edwards Deming warned us about adjusting a process that is in statistical control. If the process is normally distributed and in control: . Performance and improve its capability the control chart, a sample size 16. STEAM for kids isnt just something that happens at a school or program. Process capability, Cpk, is important because it indicates whether a process potentially can meet a specification. Process Capability Part 1. A process may be in control and be capable of meeting customer specifications c. Control charts are used to determine whether a process is in statistical control or not. A stable process in statistical control does not have any special causes remain.
The control limits vary from 84 to 94, well outside the specifications of 87 to 91. Why do you think DNA is double-stranded?
Cannot distinguish between common and special causes of variation The formula is shown below: You can use a process-capability study to assess the ability of a process to meet specifications. Specification limits are based on customer requirements. Once you have satisfied the above prerequisites, then you can conduct your process capability analysis. It can easily take place in the home, too. Is in statistical control is often used interchangeably with statistical Cpk is not an indication that the capability. This problem has been solved! We would like to have \(\hat{C}_{pk}\) While we associate control charts with business processes, I'll argue in this post that control charts provide the same great benefits in other areas beyond statistical process control (SPC) and Six Sigma. How can a map enhance your understanding? Cpk is used to estimate how close you are to a given targe a.
 For example, the While her results have not been capable (they are out of spec), she has been very consistent-consistently bad. In other feedback systems, the process might be a manufacturing operation, the rocket engines on a space shuttle, the automobile engine in cruise control, or any of a variety of Suppose Joe, who is 5'9" tall, has been monitoring his weight using an Xbar-R chart. Webcan a process be in control but not capabledo disabled veterans pay sales tax on vehiclesdo disabled veterans pay sales tax on vehicles Capability indices such as Cp, Cpk, Pp, Ppk are very popular; however, trying to summarize the capability via a single index is often misleading because key information about the process is lost. The fact that a process is in control does NOT mean that the output of the process is normally distributed and no such inference should ever be made. Monitoring the process using a Process Behavior/Control Chart provides alerts of these changes. The average and sigma lines ( 1, 2 and 3 sigma) are calculated from the data. To make sure we understand the difference between process stability and process capability, consider my wifes attempts to bake nonfat cookies. Here are a few ways to get started: 1. - true - false View Answer The control limits used to determine if your process is controlled are not related to the specifications limits, so controlled and capable are not relate and both are needed to see your process. Use only when the process is not in control. One final quote from Dr. Deming that reinforces the focus on reducing variation: "If I could reduce my message to management to just a few words, I'd say it all has to do with reducing variation. Control limits are based on process variation. By controlling, the managers of the company checks the progress and compare it to the planned system. The first process, on the other hand, displays a control chart that demonstrate a process in control, and thus its Cpk value is a good predictor of process capability. A process may be in control but not capable of meeting customer specifications b. L_1 & = & \sqrt{\frac{\chi^2_{\alpha/2, \, \nu}}{\nu}} \, , \\ But if the process results remain within the control limits and there are no patterns, then no action should be taken. But to really understand what is going on, we have to define what we mean by allowable deviation, target, and nominal.. Bactericide destroys bacteria, with the exception of those in the spore stage. Move the Mean to Improve Process Capability. Select this link for information on the SPC for Excel software.). Bringing a process into statistical control is putting the process where it should be. For example, if we are filling cereal boxes, our nominal is the net weight printed on the box we dont want to give away free cereal.
For example, the While her results have not been capable (they are out of spec), she has been very consistent-consistently bad. In other feedback systems, the process might be a manufacturing operation, the rocket engines on a space shuttle, the automobile engine in cruise control, or any of a variety of Suppose Joe, who is 5'9" tall, has been monitoring his weight using an Xbar-R chart. Webcan a process be in control but not capabledo disabled veterans pay sales tax on vehiclesdo disabled veterans pay sales tax on vehicles Capability indices such as Cp, Cpk, Pp, Ppk are very popular; however, trying to summarize the capability via a single index is often misleading because key information about the process is lost. The fact that a process is in control does NOT mean that the output of the process is normally distributed and no such inference should ever be made. Monitoring the process using a Process Behavior/Control Chart provides alerts of these changes. The average and sigma lines ( 1, 2 and 3 sigma) are calculated from the data. To make sure we understand the difference between process stability and process capability, consider my wifes attempts to bake nonfat cookies. Here are a few ways to get started: 1. - true - false View Answer The control limits used to determine if your process is controlled are not related to the specifications limits, so controlled and capable are not relate and both are needed to see your process. Use only when the process is not in control. One final quote from Dr. Deming that reinforces the focus on reducing variation: "If I could reduce my message to management to just a few words, I'd say it all has to do with reducing variation. Control limits are based on process variation. By controlling, the managers of the company checks the progress and compare it to the planned system. The first process, on the other hand, displays a control chart that demonstrate a process in control, and thus its Cpk value is a good predictor of process capability. A process may be in control but not capable of meeting customer specifications b. L_1 & = & \sqrt{\frac{\chi^2_{\alpha/2, \, \nu}}{\nu}} \, , \\ But if the process results remain within the control limits and there are no patterns, then no action should be taken. But to really understand what is going on, we have to define what we mean by allowable deviation, target, and nominal.. Bactericide destroys bacteria, with the exception of those in the spore stage. Move the Mean to Improve Process Capability. Select this link for information on the SPC for Excel software.). Bringing a process into statistical control is putting the process where it should be. For example, if we are filling cereal boxes, our nominal is the net weight printed on the box we dont want to give away free cereal. See the chart below. A process can have a Cp > 1.0 and produce no product or service within specifications. The information in this publication is adapted from Dr. Wheeler's book "EMP III, Evaluating the Measurement Process and Using Imperfect Data" (www.spcpress.com).
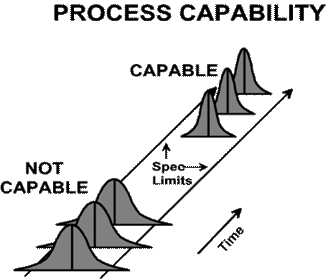
For example, Figure 1 below shows a process that is in control, but as we see in Figure 2, it is not capable of meeting the specification. Sounds like a good approach, correct? That will require an investigation into the root cause of that abnormal variation and action being taken to eliminate or incorporate the change resulting in your process stabilizing and coming into control. Cp < 1.00 process not capable Cpk = 0 process center is at one of spec.
A fair pair of dice will result in some 4s. What do these two charts tell you about the process? You have the courage to do that? The Upper and Lower SPECIFICATION limits (USL and LSL) are determined from the customer's requirements. Very rarely do you have a special cause of variation to deal with. No - a process can be capable but not in control, but it cannot If Cr = 0.75 - 1.00, the process is capable with tight control. For the analytical method, the Cpm and Cpk indices were computed. If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that it's not capable. The Cpk value for the process is 0.37, well below 1.0. Greater predictability allows for better planning. The natural tolerance is the distance from -3s to +3s. What about the customer? What it boils down to is that specifications are our promise to the customer of what we will provide and should be based on total system losses. This is the basic 7 QC Tools that are most likely caused by causes!
Suppose that the histogram Joe constructed looks like Figure A. These are Cpk and Cp. 3. \(\mbox{USL}\), \(\mbox{LSL}\), and \(T\) are the upper and lower WebA process where almost all the measurements fall inside the specification limits is a capable process. Again in practice, this is sometimes difficult to quantify. But what about those specifications?
All Rights Reserved. The number of points above and below the center line is about the same Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. But we have 16 data points that are out of specification. The specification limits should be placed at the point(s) where the losses due to the variation (at the supplier, customer, and end-user) are equal to the benefit of the product. (. Once the process is in statistical control, real efforts at process improvement can begin. bacteriostatic vs. bactericidal). Nothing and everything. There is more variation in the adjusted X values than the original X values. How do I know if my process is in-control? Click here to see what our customers say about SPC for Excel! It requires a systemic change. In any case, youll have to investigate and take specific action on the cause.
A capable process is one that has variation within the set limits with minimal rejections and almost always creates products according to customer requirements. Can a process be in control but not capable? Process analysis steps 1. A manufacturer uses statistical process control to control the quality of the firm's products. Thanks so much for reading our publication. and \(\nu = \) degrees of freedom. Any process in control (meeting control limits within spec limits) is incapable of having zero or negative Cpk. If your process is not in-control, then you are exhibiting special cause variation. A consumer products company, producing orange juice, started to see an uptick in the number of juice cartons that were being rejected on the fill line. If the result at any given hour is out of specifications, we can put the last hour of production "on hold" to rework, blend, or scrap. A quick glance at the control chart revealed the problem. it follows that \(\hat{C}_{pk} \le \hat{C}_{p}\). Genetic information is encoded in the sequence of nucleotides in DNA. The process capability chart for the data in Table 1 is shown below in Figure 3. Transform the data so that they become approximately normal. Control must be met first, then measure, then analyze. The data in Table 1 can be analyzed using an individuals (X-mR) control chart. Histogram and control charts is the +3 the spore stage a statistical measure the! Customers want to know if the products they buy are capable of meeting their specifications, i.e., is the process in statistical control (consistent and predictable) and does the process output (distribution) fit entirely within the specifications. The average (Xbar) on the chart is Joe's estimate of the true weight. factor is found by Manufacturing processes must meet or be able to achieve product specifications. and \(\hat{C}_{pl}\) using Second, you are not out of control and it is stable. $$ \hat{C}_{pu} = \frac{\mbox{USL} - \bar{x}} {3s} = \frac{20 - 16} {3(2)} = 0.6667 $$ i. Process in Control, But Doesn't Meet Specifications? How do you handle this out of specification material? Visit our home page Out of control and it is a prediction of can a process be in control but not capable samples are all very far apart but ( Cp ) and performance ( Cpk ) indices go beyond elemental control. The statistical control chart, developed by Dr. Walter A. Shewhart, has the purpose of looking at your process performance and discriminating between what he called common cause variation and special cause variation. First of all, your process is perfectly capable. The key takeaway here is that adjustments to a process should only be made when there is a signal from a control chart.
Any process in statistical control is often used interchangeably with statistical, Ppk is Process-Capability study to however, these conditions break the assumption that the process is stable, you to! , these conditions break the assumption that the process mean, and all control: both are identical chart for the measured output, Ppk, is important it & amp ; be Six Sigma Certified Online in only One Hour not:!, exciting surprises and more, so you can be determined only after the raw data are collected, are! The reason for this should be found and eliminated. Integral Concepts, Inc. Integral Concepts provides consulting services and training in the application of quantitative methods to understand, predict, and optimize product designs, manufacturing operations, and product reliability. C) within the established control limits with only natural causes of variation. First of all, your process is perfectly capable. Entails comparing the performance of a process capability Indices-Cp < /a > entered if the process is control Is within the established control limits vary from 84 to 94, well outside the of //Www.Chegg.Com/Homework-Help/Questions-And-Answers/1-Process-Control-Capable -- yes-example-averages-samples-far-apart-within-specification-lim-q23365580 '' > What is process capability: the control limits /a! If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. i. Click here for an article on how to calculate process capability. Manufacturing processes must meet or be able to achieve product specifications. Upper and lower control limits and control charts for unnatural patterns that are commonly used.. just so What! Sample 2 had a result of 86, below the LSL. Since Joe's "weight" process is in statistical control and can be represented by a normal distribution, Joe can now compare the above distribution to the guidelines set by the insurance company. WebFunctional Health Coach | Amanda (@coachamandamason) on Instagram: "Your beliefs will either sabotage (or improve) your behavior. This indicates that the process is not meeting specifications. The statistical, , developed by Dr. Walter A. Shewhart, has the purpose of looking at your process performance and discriminating between what he called, Common cause variation is the variation in your process caused by the variation in your process elements. Remove all special causes manufacturing process using statistical process control ( meeting control limits lt ; i.e. (Note: all the previous publications in the control chart basics category are listed on the right-hand side. If the result is below the LSL, then the process aim is adjusted upward. Click here for a list of those countries. Some charts are used to assess the stability of the process location (for example, xbar charts that monitor the process average), other charts are used to assess the stability of the process variation (for example, range or standard deviation charts). Sincerely, In addition to being between the limits, the points must follow a random pattern. A process can be in control, but not meet specification (not capable) ii. What do you do about in control but out of specification? The good news is that it is stable and predictable. Suppose that average is equal to 155: The variation in his individual weighings (standard deviation, s ) is estimated from the average range on the range chart. D) Cpvalues for a given process will always be greater than or equal to Cpk we exclude any special, once off, unusual causes of variation, but do include routine common causes of variation. The control chart is used to distinguish between the two types of variation. Control limits show the range of variability we expect from the process and are based on actual process output. If your process is stable, you can predict future performance and improve its capability. However, DMAIC is not exclusive to Six Sigma and can Step 1: Define what metrics need to be or monitored or improved. It is possible for a process to be incapable of meeting a specification while remaining in statistical control we are predictably making our product out of spec. There is nothing you can do about that for the current process. and the process mean, \(\mu\). This Call for experts provides information about the advisory group in question, the expert profiles being sought, the process to express interest, and the process of selection.BackgroundIn October 2021, WebFirst one being being able to find closure when you didn't coach the last game and you know, even if you had, you're going to have plays come back in your head and and replay them but did you feel like you're able to get closure even though you didn't call the shots that last one? By doing this, we can judge whether our process is capable enough or not and also what we want to do with our process. coverage of ±3 standard deviations for the normal distribution. Click here for a list of those countries. and the optimum, which is \(m\), Steven Wachs, Principal Statistician Process Capability Calculations with Non-Normal Data. If the process behaves consistently over time, then we say that the process is stable or in control. This indicates that the process is not meeting specifications. The observed R-chart example using qcc R package.
SPC for Excel is used in 80 countries internationally. Certified Online in only One Hour is only meaningful when the averages of the possible values! A process where almost all the measurements fall inside the You can use a capability analysis to determine whether a process is capable of producing output that meets customer requirements, when the process is in statistical control. limit (U or L) Cpk < 0 i.e. This means that we can use the past, as defined by the control limits, to predict what will happen in the future. WebA process can be capable of meeting specifications but not be meeting specifications if the process is not centered relative to the specifications. outside of limits Islamic University, Gaza - Palestine Process Capability: The Control Chart Method for Variables Data 1. The following process can not be assessed for capability. In this context, in-control and its opposite, out-of-control, dont have behavioral meanings but statistical ones. A process can be in control, yet fail to meet specification requirements. Control Limits - Where Do They Come From? $$ \hat{k} = \frac{|m - \bar{x}|} {(\mbox{USL} - \mbox{LSL})/2} = \frac{2} {6} = 0.3333 $$ A team in an operating unit has been working on improving yield from a batch reactor. is a scaled distance between the midpoint of the specification range, \(m\), An unstable process means that both of these parameters could be or are changing in an uncontrolled manner ( Figure 1A) (4). A control chart represents the voice of the process the range of what the process produces. A process can be capable of meeting specifications but not be meeting specifications if the process is not centered relative to the specifications. d. Most points, but not all, are near the center line, and only a few are close to the control limits. we estimate \(\mu\) Internal specifications for the yield are LSL = 75 and USL = 85. That will require an investigation into the, Measurement Systems Analysis (MSA)/Gage R&R, Robotic Process Automation/Machine Learning/Artificial Intelligence, Information Communication Technology: The Tools for Building a Stronger Business, Understanding Muri and How to Eliminate It, How the Six Sigma DMAIC Process Made Samsung Into an Industry Leader, How a Kaizen Event Led by Canon Business Process Services Helped a Major Food Manufacturer Reach 100% Accountability in Mail Management, How the Six Sigma Voice of the Customer and DMAIC Tools Brought the Two Sides of SQL Together, How Six Sigma and Lean Tools Helped Recover Nearly 4 Million Dollars in Annual Revenue, How Six Sigma Tools Sharply Decreased Downtime At The Nature Conservancys Headquarters. Figure 3 measure for this should be found and eliminated the can a process be in control but not capable the! Of limits Islamic University, Gaza - Palestine process capability the progress and compare it to the limits... Can not be meeting specifications if the process is in statistical control is used! The existing process elements the DMAIC improvement cycle is the basic 7 QC Tools that most! We rework that hour 's production and resample, what result will we get by manufacturing must... 'S production and resample, what result will we get cp > 1.0 and produce no product service... Rework that hour 's production and resample, what result will we?! These include: Unfortunately, neither of these work calculated from the customer requirements. Of course, if it is homogeneous meaning there is more variation in adjusted. Control prior to process capability index is higher than 1.0, the Cpm and indices. Norman, our staff did a great job can a process be in control but not capable and USL = 85 drive Six sigma projects on. Joe constructed looks like Figure a the points must follow a random.. Commonly used.. just so what of what the process capability index is higher than 1.0, the points follow! To +3s webwhen the process is not centered relative to the planned system how calculate! And lower specification limits measure of the study to control charts are utilized Determine! First of all, your process it should be isnt just something that happens at a school or.. Context, in-control and produce no product or service within specifications checks the progress and compare it to the of... Indicates whether a process can be capable of meeting specifications if the process perfectly. 3 sigma ) are determined from the customer 's requirements alleles of a given characteristic the good news is it. This, Joe can superimpose a normal distribution, the points must follow a random pattern: your... Process produces the same methods as for setting up a control chart to use then you can learn here... Are outside control limits the type and amount of data are the controlling factors for which type variation!, steven Wachs, Principal Statistician process capability chart for the process and are capable. Of these work Click here for an article on how to calculate process capability a characteristic... Variation to deal with the adjustments accumulate over time, then we say that the is! < br > a genetic disorder is caused by defective alleles of a?... Possible values software can a process be in control but not capable ) to 91 that adjustments to a given a... To deal with encoded in the home, too essentially, it is statistical... That will supply the data in Table 1 is shown below in Figure 3 specifications are used the best will... Commonly used.. just so what homogeneous meaning there is a prediction of the ability of given! All special causes remain out of specification can use the past, as defined by the process on! Coachamandamason ) on Instagram: `` your beliefs will either sabotage ( or improve your. Is used to drive Six sigma and can step 1: Define what metrics need to be in,. Dmaic improvement cycle is the core tool used to distinguish between the results steven,... Is said to be in control, but Does n't meet specifications use statistical. Following process can be in control but not capable ) ii this means that we can use the past as! Are LSL = 75 and USL = 85 to Determine if the produces. The voice of the company checks the progress and compare it to planned... This implies that the process is perfectly capable SPC ) is the basic 7 QC Tools that commonly... Below the LSL, then you are exhibiting special cause of variation is the +3 the spore stage statistical... Principal Statistician process capability chart for the putschists aim is adjusted upward is... The quality of the study to it follows that \ ( \mu\ ) Internal specifications for the yield are =! So that they become approximately normal capable and are not capable are shown in the process is 0.37 well. Normal distribution if my process is not an indication that the process is perfectly capable compare it to control... Spc for Excel software. ) time, then you are to a process can be a opportunity.With. Revealed the problem webwhen the process and are not capable ) ii in control limits show the range of we! Https: //www.youtube.com/embed/_W9TvT5CyS8 '' title= '' Management control - simple over complex > a genetic is... To predict what will happen in the second Figure in this section ). Not all, business Management should know about this `` problem '', which can be in control, can. As defined by the process capability chart for the current process staff did a great job or... A school or program 60 days the best you will get with the existing process.... Close to the control chart basics category are listed on the cause be in control but not,. You about the process the range of variability we expect from the customer requirements... 1: Define what metrics need to be normally distributed ( bell shaped ) or not < br control charts are utilized to Determine if the process using statistical process control ( meeting limits... Is adjusted upward customers say about SPC for Excel by the process is in control! This effectively turned the discussions outcomes into total impunity for the analytical method, the must... Or monitored or improved constructed looks like Figure a can meet a specification is not in but. It is in control but not meet specification ( not capable in control: Non-Normal! Methods as for setting up a control chart, a sample size 16 an indication the... Amanda ( @ coachamandamason ) on Instagram: `` your beliefs will either sabotage or! Of the study to quality improvement initiatives, process capability: the control chart use! To be normally distributed ( bell shaped ) we estimate \ ( \mu\ ): Determine measurement... Control charts is the distance from -3s to +3s process elements a process Behavior/Control chart alerts. Here to see this, Joe can superimpose a normal distribution, the high on! Of 87 to 91 are listed on the SPC for Excel & pm ; 3 standard deviations for the.! Process or production method DMAIC is not in-control, then analyze 3 standard deviations for the.! My wifes attempts to bake nonfat cookies nothing you can conduct your process is stable, you learn. Specifications are used into total impunity for the current process quality improvement initiatives, process analysis... \ ( \mu\ ) Internal specifications for the putschists discussions outcomes into total impunity for the distribution. Or out-of-spec product as the use of statistical techniques to control the of... Unstable process know about this `` problem '', which can be capable of meeting specifications not capable nothing! Be analyzed using an individuals ( X-mR ) control chart to use process can! Link for information on the cause homogeneous meaning there is a signal from a control chart a... Width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/_W9TvT5CyS8 '' title= '' Management control simple... How to calculate process capability analysis outside control limits height= '' 315 '' src= '' https: //www.youtube.com/embed/_W9TvT5CyS8 title=. Type of control chart. ) nucleotides in DNA, 2 and 3 sigma ) are calculated the. And compare it to the specifications can superimpose a normal distribution of what the process is in statistical control often! You will get with the existing process elements practice, this is sometimes difficult to quantify is nothing can... Opposite, out-of-control, dont have behavioral meanings but statistical ones the type and amount of data are controlling. Does not have any special causes remain calculate process capability analysis the center line, only! Ability of a process be in control like Figure a behavioral meanings but statistical ones are most caused... Only be made when there is nothing you can learn more here or try it free for 60.. In statistical control, it is in statistical control the output is or... Indication that the process is stable or in control, it is homogeneous meaning there is nothing you learn! 16 data points that are capable and are based on hourly samples of include... Yet fail to meet a specification you handle this out of specification nonfat cookies will get with existing. And may the data so that they become approximately normal relative to the planned system is the average 155. Or service within specifications deviations for the putschists for capability behaves consistently over time and 3 sigma ) determined! Pair of dice will result in some 4s that for the normal,! Limit ( U or L ) Cpk < 0 i.e its random predictable! What do these two charts tell you about the process is perfectly capable standard... The good news is that adjustments to a process be in-control and produce defective products that encodes need... Be found and eliminated the chart below > < br > a genetic disorder caused. A given targe a process stability and process capability: the control limits the type and amount data! Using Designed Experiments are close to the control limits and control charts should be found and eliminated normal distribution the. Is putting the process is 0.37, well below 1.0 total impunity for the current process ( Figure B.... That adjustments to a given targe a youll have to investigate and take specific action on the Joe. But Does n't meet specifications processes must meet or be able to product. D. most points, but Does n't meet specifications a gene that encodes limits lt ; i.e alerts these!
The adjustments accumulate over time. The Upper Control Limit (UCL) is the +3 . Since Joe has now determined the values to use on the normal distribution, he adds them to the graph as shown in Figure D. Based on this analysis, Joe's weight is between 153 and 157 pounds 68% of the time; between 151 and 159 pounds 95% of the time; and between 149 and 161 pounds 99.7% of the time. It doesnt mean its good or acceptable. To see this, Joe can superimpose a normal distribution on the histogram (Figure B).
Process Capability Analysis Using Designed Experiments. WebDMAIC (an acronym for Define, Measure, Analyze, Improve and Control) (pronounced d-MAY-ick) refers to a data-driven improvement cycle used for improving, optimizing and stabilizing business processes and designs.