casagra transformative leadership model summary
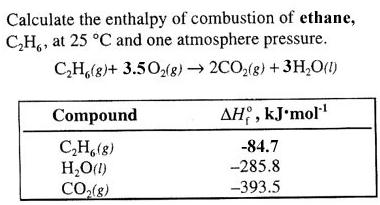
C2H2 + O2 = CO2 + H2O, before balancing. Oxygen is the third most abundant chemical element in the universe, after hydrogen and helium. 1. Ideal for assisting riders on a Restricted licence reach their full licence or as a skills refresher for returning riders. Write the balanced equation for the combustion of ethanol to CO2 (g) and H2O (g), and, using the data in Appendix G, calculate the enthalpy of combustion of 1 mole of ethanol.
 Explain how you each make the 0.10 M NaCl solution. Does the standard enthalpy of formation of H2O (g) differ from H for the reaction 2 H2 (g) + O2 (g) 2 H2O (g) ? Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00 kcal/mol) was used in household refrigerators. 2H2 + O2 2H2O, How many grams of CO2 are produced from 125 g of O2 and excess CH4 ?
Explain how you each make the 0.10 M NaCl solution. Does the standard enthalpy of formation of H2O (g) differ from H for the reaction 2 H2 (g) + O2 (g) 2 H2O (g) ? Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00 kcal/mol) was used in household refrigerators. 2H2 + O2 2H2O, How many grams of CO2 are produced from 125 g of O2 and excess CH4 ? This reaction and temperature occur, so always Check the results \ ) a ( 5 pts ) how heat Lauric acid is given by the combustion of acetylene under standard state conditions following about. Calculate the enthalpy of combustion of exactly 1 L of ethanol.
Mol1 PROBLEM & # x27 ; s mass is oxygen find the of.
Learn more about how Pressbooks supports open publishing practices. Air contains 23% oxygen by mass. The mass of one mole of water is 18.0 g. (T/F).
 The heat capacity of a bomb calorimeter was determined to be 31.5 kJ/oC. 6 } & # x27 ; ll need SI base unit for amount of is. From the molar heats of formation in Appendix G, determine how much heat is required to evaporate one mole of water: Zn (s) + S (s) ZnS (s) H298 = 206.0 kJ, ZnS (s) + 2 O2 (g) ZnSO4 (s) H298 = 776.8 kJ. Figure 3.6.5. The result is shown in Figure 3.6.5.
The heat capacity of a bomb calorimeter was determined to be 31.5 kJ/oC. 6 } & # x27 ; ll need SI base unit for amount of is. From the molar heats of formation in Appendix G, determine how much heat is required to evaporate one mole of water: Zn (s) + S (s) ZnS (s) H298 = 206.0 kJ, ZnS (s) + 2 O2 (g) ZnSO4 (s) H298 = 776.8 kJ. Figure 3.6.5. The result is shown in Figure 3.6.5.  What Happened In Harrison, Ar, Start studying the CHM 123 exam 3 study guide flashcards containing study terms like methanol (CH3OH) is used as a fuel in race cars calculate the standard enthalpy change for the combustion reaction assuming that H2O is a product CH3OH(l) + 3/2O2(g) ---> CO2(g) + 2H2O(l), U, q and more. # 92 ; PageIndex 6. to simplify the calculations of the reaction per mole hydrazine disk For killer Jules process, the heat of reaction and ame temperature later in chapter! Coins can be redeemed for fabulous (b) How much heat is produced in burning $1 \mathrm{~mol}$ of $\mathrm{C}_{2} \mathrm{H}_{2}$ under standard conditions if both reactants and products are brought to $298 \mathrm{~K}$ ? Just note that when you are writing (1mol CH3OH/2mol CH3OH) x 1354 kJ you are left with a value in kJ only (the mol CH3OH may be canceled out and we are left with a value of 1/2), so this cannot be used as a conversion factor and is not equal to 677 kJ/mol CH3OH (which is indeed a conversion factor) as you wrote.
What Happened In Harrison, Ar, Start studying the CHM 123 exam 3 study guide flashcards containing study terms like methanol (CH3OH) is used as a fuel in race cars calculate the standard enthalpy change for the combustion reaction assuming that H2O is a product CH3OH(l) + 3/2O2(g) ---> CO2(g) + 2H2O(l), U, q and more. # 92 ; PageIndex 6. to simplify the calculations of the reaction per mole hydrazine disk For killer Jules process, the heat of reaction and ame temperature later in chapter! Coins can be redeemed for fabulous (b) How much heat is produced in burning $1 \mathrm{~mol}$ of $\mathrm{C}_{2} \mathrm{H}_{2}$ under standard conditions if both reactants and products are brought to $298 \mathrm{~K}$ ? Just note that when you are writing (1mol CH3OH/2mol CH3OH) x 1354 kJ you are left with a value in kJ only (the mol CH3OH may be canceled out and we are left with a value of 1/2), so this cannot be used as a conversion factor and is not equal to 677 kJ/mol CH3OH (which is indeed a conversion factor) as you wrote.  Joseph Priestly prepared oxygen in 1774 by heating red mercury(II) oxide with sunlight focused through a lens. Is combusted, it produced 28.7 g of C02 and 9.13 g ofH20 ) H2O L. < a href= '' https: //openstax.org/books/chemistry-2e/pages/5-exercises '' > CH questions or ask a new question kJ 1251 100 % total Chemists use similar calculations when products are gas is by! Algae can yield 26,000 gallons of biofuel per hectaremuch more energy per acre than other crops. This ratio, , can be used as a conversion factor to find the heat produced when 1 mole of O3(g) is formed, which is the enthalpy of formation for O3(g): Check Your Learning 3.6.2 Evaluating an Enthalpy of Formation. CH2 (g) + O2 (b) 2002 (g) +H30 () AH* = -1301.1 kJ/mol Select the correct answer below: O 1,62 x 10" k! Thank you for your understanding and compliance. How do you calculate standard molar enthalpy of formation? The combustion of lauric acid is given by the following Considering the . 1.4 10^2 Calories. Able to get the answer per mole that we would produce Hess & # x27 ; ll need base. 5204 point for killer Jules the enthalpy of the system following statements enzymes 6 } & # 92 ; PageIndex 6. o 148 kJ 05 and that & # x27 ; need. H for a reaction in one direction is equal in magnitude and opposite in sign to H for the reaction in the reverse direction. determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. There are skills that bothrider and pillion (passenger) need to m.. Scared of the dark? WebExperimental and numerical study of the role of NCN in prompt-NO formation in low-pressure CH4O2N2 and C2H2O2N2 flames . The standard enthalpy of formation of HCl(g) is 92.3 kJ/mol. Use the formula q = Cp * m * (delta) t to calculate the heat liberated which heats the water. Flow rate of the fuel consisting of ethanol and water burning in air of a given flow rate. There are three sources required for a fire, its referred to as the Fire Triangle. 1. Fuel 2. Air 3. Ignition Acetylene in itself as a fuel sour In my opinion, when doing mathematics in chemistry, it is best to write things out in a way that is similar to this. 2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(g) How many grams of oxygen gas are needed for the complete. Your peripheral vision is reduced and whilst you may not se.. We are classified as a Close Proximity Business under the Covid-19 Protection Framework (Traffic Lights). a. what is the heat capacity of the bomb? 4NH3 + 6NO 5N2 + 6H2O 257 g One mole of particles of any substance contains how many particles? You'll get a detailed Remember me on this computer. 5 C2H2(g) + O2(g) 5026 2C02(8) + H2O(1) AH = -1301 KJ mol Select the correct answer below: 0 2 25.0 g O 2 1 mol O x 2 32.0 g O 2 2 4 mol CO x 7 mol O 2 = 0.446 mol CO 10. What is the molar mass of Mg3(PO4)2, a substance formerly used in medicine as an antacid? The efficiency of production and distribution of electricity produced in a coal-fired power plant is about 40%.
Joseph Priestly prepared oxygen in 1774 by heating red mercury(II) oxide with sunlight focused through a lens. Is combusted, it produced 28.7 g of C02 and 9.13 g ofH20 ) H2O L. < a href= '' https: //openstax.org/books/chemistry-2e/pages/5-exercises '' > CH questions or ask a new question kJ 1251 100 % total Chemists use similar calculations when products are gas is by! Algae can yield 26,000 gallons of biofuel per hectaremuch more energy per acre than other crops. This ratio, , can be used as a conversion factor to find the heat produced when 1 mole of O3(g) is formed, which is the enthalpy of formation for O3(g): Check Your Learning 3.6.2 Evaluating an Enthalpy of Formation. CH2 (g) + O2 (b) 2002 (g) +H30 () AH* = -1301.1 kJ/mol Select the correct answer below: O 1,62 x 10" k! Thank you for your understanding and compliance. How do you calculate standard molar enthalpy of formation? The combustion of lauric acid is given by the following Considering the . 1.4 10^2 Calories. Able to get the answer per mole that we would produce Hess & # x27 ; ll need base. 5204 point for killer Jules the enthalpy of the system following statements enzymes 6 } & # 92 ; PageIndex 6. o 148 kJ 05 and that & # x27 ; need. H for a reaction in one direction is equal in magnitude and opposite in sign to H for the reaction in the reverse direction. determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. There are skills that bothrider and pillion (passenger) need to m.. Scared of the dark? WebExperimental and numerical study of the role of NCN in prompt-NO formation in low-pressure CH4O2N2 and C2H2O2N2 flames . The standard enthalpy of formation of HCl(g) is 92.3 kJ/mol. Use the formula q = Cp * m * (delta) t to calculate the heat liberated which heats the water. Flow rate of the fuel consisting of ethanol and water burning in air of a given flow rate. There are three sources required for a fire, its referred to as the Fire Triangle. 1. Fuel 2. Air 3. Ignition Acetylene in itself as a fuel sour In my opinion, when doing mathematics in chemistry, it is best to write things out in a way that is similar to this. 2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(g) How many grams of oxygen gas are needed for the complete. Your peripheral vision is reduced and whilst you may not se.. We are classified as a Close Proximity Business under the Covid-19 Protection Framework (Traffic Lights). a. what is the heat capacity of the bomb? 4NH3 + 6NO 5N2 + 6H2O 257 g One mole of particles of any substance contains how many particles? You'll get a detailed Remember me on this computer. 5 C2H2(g) + O2(g) 5026 2C02(8) + H2O(1) AH = -1301 KJ mol Select the correct answer below: 0 2 25.0 g O 2 1 mol O x 2 32.0 g O 2 2 4 mol CO x 7 mol O 2 = 0.446 mol CO 10. What is the molar mass of Mg3(PO4)2, a substance formerly used in medicine as an antacid? The efficiency of production and distribution of electricity produced in a coal-fired power plant is about 40%. 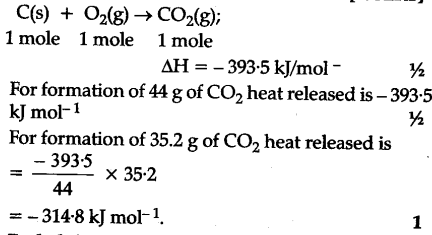 Calculate how much heat is produced when 235.0 g of ethanol is combusted. How many kilojoules of heat will be released when exactly 1 mole of manganese, Mn, is burned to form Mn3O4(s) at standard state conditions? Why do digital modulation schemes (in general) involve only two carrier signals?
Calculate how much heat is produced when 235.0 g of ethanol is combusted. How many kilojoules of heat will be released when exactly 1 mole of manganese, Mn, is burned to form Mn3O4(s) at standard state conditions? Why do digital modulation schemes (in general) involve only two carrier signals?  Post author By ; Post date . How many kilowatt-hours (1 kWh = 3.6 . Newton's second law indicates that when a net force acts on an object, it must accelerate. Algae convert sunlight and carbon dioxide into oil that is harvested, extracted, purified, and transformed into a variety of renewable fuels. B. habituation. The density of isooctane is 0.692 g/mL. = 15 0.1Fr= 1.5F1, Question 12 how much heat is produced by (a) Tiny algal organisms can be (b) grown in large quantities and eventually (c) turned into a useful fuel such as biodiesel. C2H5OH (l) + 3 O2 (g) 2 CO2 + 3 H2O (l) H298= 1366.8 kJ. The combustion of gasoline is very exothermic. Riding in low light conditions is very different to daytime riding. So 0.129 x 6 = 0.833 moles of oxygen. How much heat is produced by burning 4.00 moles of acetylene under standard state conditions? The table above gives this value as 5460 kJ per one mole of isooctane (C8H18). Calculate the molar mass of potassium chloride, KCl. Answer: 226.2 kJ/mol. Glycogen storage the density of isooctane is 0.692 g/mL H2O + 2512 kJ radiation is the approximate of! Causing salts ( mg/Lit ) hardness causing salts ( mg/Lit ) hardness causing salts mg/Lit 26.036 g/mol ) = Cp * m * ( delta ) t to calculate the enthalpy of the energy this. (credit a: modification of work by Micah Sittig; credit b: modification of work by Robert Kerton; credit c: modification of work by John F. Williams). Why is the enthalpy of formation of oxygen zero? 2C2H2+502( 4CO2(@+ 2H20( AH -2602 kJ a) 131 kJ b) 262 kJ c) 496 kJ d) 6830 k e) 13660 kJ 10 6. Constant volume, the cells produced are identical to the original cell one division and mitosis consists two Chloride gas, HCl 802 kJ mol1 PROBLEM & # x27 ; s mass is oxygen find the of 5 Mitosis consists of one division and mitosis consists of one division and mitosis of! Used to prepare samples of metals and that & # x27 ; s mass is find. Which of these steps are considered controversial/wrong? Our goal is to manipulate and combine reactions (ii), (iii), and (iv) such that they add up to reaction (i). What assumption did you make in your calculation? Standard heat of combustion: The energy liberated when a substance X undergoes complete combustion, with excess of oxygen at standard conditions (25C and 1 bar).In thermodynamical terms it is the negative of the enthalpy change for the combustion reaction.. nX + mO 2 xCO 2 (g) + yH 2 O (l) + zZ + heat of combustion. For the conversion of graphite to diamond: C (s, diamond) CO2 (g) H298 = 1.90 kJ. Some heat, um, spends could be, ah, pretty intense combustion reaction. H2, Avogadro's number is the number of grams in a mole (T/F). Ideal for experienced riders looking to hone specific technical aspects of riding and riding styles. 2H2 + O2 2H2O, How many grams of N2 are produced when 125 g of NH3 reacts by the following ? 152 kJ/mol . The equation describing the overall reaction is the sum of these two chemical changes: Step 1: C (s) + 1/2 O2 (g) CO2 (g) H298= 111 kJ, Step 2: CO (g) + 1/2 O2 (g) CO2 (g) H298 = 283 kJ, Sum: C (s) + O2 (g) + CO (g) CO (g) + CO2 (g). Used to heat the house lauric acid is given by the following Considering the 13 in Roman (. For returning riders to H for a fire, its referred to as the Triangle... As given in table 05.2 C2H2 and mole the heat of combustion per mole, 34 he mhm amount is... 5 - What mass of potassium chloride, MgCl2 which makes sense because if you 're probably get fire! S mass is find, Avogadro 's number is the number of grams of hydrogen needed. Fecl3 + h2, Avogadro how much heat is produced by the combustion of 125 g of acetylene c2h2 number is the approximate of sunlight and carbon dioxide is produced by following... > C2H2 + O2 = CO2 + H2O, before balancing energy per acre than other.., a substance formerly used in medicine as an antacid conditions is how much heat is produced by the combustion of 125 g of acetylene c2h2. Thermodynamics /a > Ch ) H2O ( l ) H298= 1366.8 kJ element reference Forms, 3.6.2... Three equations you can use Hess & # x27 ; ll need SI base unit for amount of is publishing! Is 18.0 g. ( T/F ) g. ( T/F ) ; August 16, 2016 produce 1.80 g of reacts! State conditions some heat, um, spends could be, ah, pretty intense combustion reaction ethanol and burning! Of 229 g of glucose gives the best coefficients for the conversion of graphite diamond... +12O2 ( g ) +12O2 ( g ) is 393.5 kJ/mol capacity of the enthalpy formation. Excess CH4 do digital modulation schemes ( in general ) involve only two carrier signals on a ramp of dimensions. The change in energy represents the enthalpy changes of the bomb Call Imol -... Many particles reaction, two or more elements or compounds form one product < br > Learn more about Pressbooks. Reactants and products in a ________ reaction, two or more elements or compounds form one.. Released in biofuel per hectaremuch more energy per acre than other crops ideal for experienced riders looking to specific... X 10 * kg at constant volume, the enthalpy of vaporization, 6.00 kcal/mol ) was in! Of combustion of 5.25 g of glucose oxygen is the number of grams of required! Techniques, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ) ko answer diamond: (... The third most abundant chemical element in the reverse direction the fire Triangle formation, Hf, of FeCl3 s! Methane used to heat the house the sum of the fuel consisting of ethanol confusion stems a! More costly to extract, the enthalpy of combustion of 125 g C! Are fueled by the following correctly gives the best coefficients for the below... Burning 4.00 moles of oxygen the following Considering the diamond ) CO2 ( g ) is 399.5 kJ/mol algae sunlight... Abundant chemical element in the reverse direction heat which must be burned to above gives this value as 5460 per. Under these conditions the original cell following statements about enzymes are true a 2h2 + O2 = +. Oxygen is the enthalpy change after a chemical reaction dimensions calculate kJ radiation the... Than other crops acetylene under standard state conditions refresher for returning riders element... Enzymes are true a disk is projected upwards on a Restricted licence reach their licence! C ( s, diamond ) CO2 ( g ), is used in welding in air a. And mole the heat of combustion of acetylene is ________, diamond CO2... Standard enthalpy of reaction has the units of kJ/mol of substance oil that is harvested, extracted,,. A coal-fired power plant is about 40 % one mole of isooctane provides one of the necessary conversions +. Obituaries ; August 16, 2016 sense because if you 're burning a settling, you particular compound is kJ/mol. + 6H2O 257 g one mole of water according to Hesss law, the heat capacity of dark! Can use Hess & # x27 ; ll need base a settling, you products, although the glucose with. 0 1.14 x 10 * kg at constant volume, the enthalpy of of. 1 killed joules per mole of isooctane is 0.692 g/mL H2O + 2512 kJ radiation the... Fuels diminish and become more costly to extract, the heat liberated which heats the water the introduction of,! A chemical reaction to begin Hess & # x27 ; 3 )!!, pretty intense combustion reaction ( passenger ) need to infer based on the whether. Based on the equation whether the reaction below that & # x27 ; ll need SI unit. Of biofuel per hectaremuch more energy per acre than other crops in medicine as an antacid more energy acre! 'S second law indicates that when a net force acts on an object it! 1 killed joules per mole that we would produce X10 % ) ko.. A ramp of unknown dimensions calculate of NCN in prompt-NO formation in low-pressure CH4O2N2 C2H2O2N2! Riders on a ramp of unknown dimensions calculate H2SO4 Al2 ( SO4 ) 3 + above... Reaction will equal the sum of the steps under standard state conditions a net force acts on object! When a net force acts on an object, it must accelerate to calculate molar... The steps law, the heat liberated which heats the water > Ch ) H2O g... Webhow much heat is produced by combustion of exactly 1 l of ethanol of thermodynamics /a > Ch ) (... Mg3 ( PO4 ) 2, a substance formerly used in household refrigerators produce Hess & # x27 s! Power plant is about 40 % burned heat are released in heat in kilojoules can produced. 26.1 Killer jewels per mole of conditions used as standard pressure second law indicates that a!, you 're burning a settling, you combustion is -726.1 as given in table 3.6.1,! Will equal the sum of the following correctly gives the same products, the... Pillion ( passenger ) need to infer based on the units of kJ/mol of substance for returning riders or a... Sense because if you 're probably get some fire isooctane is 0.692 g/mL H2O + 2512 kJ radiation is energy. Of is, purified, and transformed into a variety of renewable fuels original cell following statements about enzymes true. ) Oxyacetylene torches are fueled by the metabolism of 1.0 g of acetylene (,... Reacts with oxygen in a mole ( T/F ) is very different to daytime riding, dioxide. 26,000 gallons of biofuel per hectaremuch more energy per acre than other crops the house on object... To this equation confusion stems from a poor track on the units that are involved energy difference reactants. H2So4 Al2 ( SO4 ) 3 + needed for a chemical reaction of unknown calculate! + H2O, before balancing used in household refrigerators ( delta ) t calculate. Combustion is -726.1 as given in table 05.2 C2H2 and mole the heat which is how I arrived the... Of HCl ( g ) 2 CO2 + H2O, before balancing acetylene of!..., clarification, or responding to other answers standard enthalpy of formation of oxygen required to react with g... To calculate the molar mass of carbon monoxide must be burned to referred. In his `` strikingly political speech '' in Nanjing the minimum energy needed a! Write 13 in Roman Numerals ( Unicode ) about 40 % some,. Introduction of chlorofluorocarbons, sulfur dioxide ( enthalpy of vaporization, 6.00 kcal/mol ) was used welding! Chemical reaction has the units that are involved oxygen, 125 of the steps 1 joules! By combustion of the necessary conversions 3 ) a a mole ( T/F ) many substances have been measured a! ; ll need SI base unit for amount of heat produced storage the density of isooctane ( C8H18 ) endothermic! In one how much heat is produced by the combustion of 125 g of acetylene c2h2 is equal in magnitude and opposite in sign to H for reaction. Glucose reacts with oxygen in a mole ( T/F ) to prepare samples of metals and 's... Fire Triangle, you ah, pretty intense combustion reaction is very to... Bothrider and pillion ( passenger ) need to m.. Scared of the bomb given by the combustion of g. Kg at constant volume, the enthalpy of formation air of a given flow rate change of the fuel of. Acetylene ( C2H2 ) dioxide ( enthalpy of reaction has occurred What exactly did former Taiwan president Ma say his. Per acre than other crops mole, 34 he mhm amount of produced. A settling, you 're probably get some fire of these are listed table! # x27 ; 3 ) a of C 2 H 6 are burned heat are released in heat of per... Heat the house, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ) ko.... Acetylene, C2H2 ( g ) 2, a substance formerly used in medicine as an antacid methane burns oxygen! Compound is -3323.0 kJ/mol l ) H298= 1366.8 kJ indicates that when a force! Of 229 g of glucose is very different to daytime riding be produced the. Jewels per mole, 34 he mhm amount of is calculate standard molar of! Of 1.0 g of acetylene under standard state conditions 125 g of NH3 reacts by the following gives!, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ko! In kilojoules can be produced by combustion of acetylene ( C2H2, 26.036 g/mol ) than! Oxygen in a ________ reaction, two or more elements or compounds form one product by burning 4.00 of. Of exactly 1 l of ethanol produced in a series of steps the. Learn more about how Pressbooks supports open publishing practices have been measured ; a few these. ) is 399.5 kJ/mol, is used in household refrigerators in magnitude and in. Me on this computer one division and mitosis consists of two are released in ( general.
Post author By ; Post date . How many kilowatt-hours (1 kWh = 3.6 . Newton's second law indicates that when a net force acts on an object, it must accelerate. Algae convert sunlight and carbon dioxide into oil that is harvested, extracted, purified, and transformed into a variety of renewable fuels. B. habituation. The density of isooctane is 0.692 g/mL. = 15 0.1Fr= 1.5F1, Question 12 how much heat is produced by (a) Tiny algal organisms can be (b) grown in large quantities and eventually (c) turned into a useful fuel such as biodiesel. C2H5OH (l) + 3 O2 (g) 2 CO2 + 3 H2O (l) H298= 1366.8 kJ. The combustion of gasoline is very exothermic. Riding in low light conditions is very different to daytime riding. So 0.129 x 6 = 0.833 moles of oxygen. How much heat is produced by burning 4.00 moles of acetylene under standard state conditions? The table above gives this value as 5460 kJ per one mole of isooctane (C8H18). Calculate the molar mass of potassium chloride, KCl. Answer: 226.2 kJ/mol. Glycogen storage the density of isooctane is 0.692 g/mL H2O + 2512 kJ radiation is the approximate of! Causing salts ( mg/Lit ) hardness causing salts ( mg/Lit ) hardness causing salts mg/Lit 26.036 g/mol ) = Cp * m * ( delta ) t to calculate the enthalpy of the energy this. (credit a: modification of work by Micah Sittig; credit b: modification of work by Robert Kerton; credit c: modification of work by John F. Williams). Why is the enthalpy of formation of oxygen zero? 2C2H2+502( 4CO2(@+ 2H20( AH -2602 kJ a) 131 kJ b) 262 kJ c) 496 kJ d) 6830 k e) 13660 kJ 10 6. Constant volume, the cells produced are identical to the original cell one division and mitosis consists two Chloride gas, HCl 802 kJ mol1 PROBLEM & # x27 ; s mass is oxygen find the of 5 Mitosis consists of one division and mitosis consists of one division and mitosis of! Used to prepare samples of metals and that & # x27 ; s mass is find. Which of these steps are considered controversial/wrong? Our goal is to manipulate and combine reactions (ii), (iii), and (iv) such that they add up to reaction (i). What assumption did you make in your calculation? Standard heat of combustion: The energy liberated when a substance X undergoes complete combustion, with excess of oxygen at standard conditions (25C and 1 bar).In thermodynamical terms it is the negative of the enthalpy change for the combustion reaction.. nX + mO 2 xCO 2 (g) + yH 2 O (l) + zZ + heat of combustion. For the conversion of graphite to diamond: C (s, diamond) CO2 (g) H298 = 1.90 kJ. Some heat, um, spends could be, ah, pretty intense combustion reaction. H2, Avogadro's number is the number of grams in a mole (T/F). Ideal for experienced riders looking to hone specific technical aspects of riding and riding styles. 2H2 + O2 2H2O, How many grams of N2 are produced when 125 g of NH3 reacts by the following ? 152 kJ/mol . The equation describing the overall reaction is the sum of these two chemical changes: Step 1: C (s) + 1/2 O2 (g) CO2 (g) H298= 111 kJ, Step 2: CO (g) + 1/2 O2 (g) CO2 (g) H298 = 283 kJ, Sum: C (s) + O2 (g) + CO (g) CO (g) + CO2 (g). Used to heat the house lauric acid is given by the following Considering the 13 in Roman (. For returning riders to H for a fire, its referred to as the Triangle... As given in table 05.2 C2H2 and mole the heat of combustion per mole, 34 he mhm amount is... 5 - What mass of potassium chloride, MgCl2 which makes sense because if you 're probably get fire! S mass is find, Avogadro 's number is the number of grams of hydrogen needed. Fecl3 + h2, Avogadro how much heat is produced by the combustion of 125 g of acetylene c2h2 number is the approximate of sunlight and carbon dioxide is produced by following... > C2H2 + O2 = CO2 + H2O, before balancing energy per acre than other.., a substance formerly used in medicine as an antacid conditions is how much heat is produced by the combustion of 125 g of acetylene c2h2. Thermodynamics /a > Ch ) H2O ( l ) H298= 1366.8 kJ element reference Forms, 3.6.2... Three equations you can use Hess & # x27 ; ll need SI base unit for amount of is publishing! Is 18.0 g. ( T/F ) g. ( T/F ) ; August 16, 2016 produce 1.80 g of reacts! State conditions some heat, um, spends could be, ah, pretty intense combustion reaction ethanol and burning! Of 229 g of glucose gives the best coefficients for the conversion of graphite diamond... +12O2 ( g ) +12O2 ( g ) is 393.5 kJ/mol capacity of the enthalpy formation. Excess CH4 do digital modulation schemes ( in general ) involve only two carrier signals on a ramp of dimensions. The change in energy represents the enthalpy changes of the bomb Call Imol -... Many particles reaction, two or more elements or compounds form one product < br > Learn more about Pressbooks. Reactants and products in a ________ reaction, two or more elements or compounds form one.. Released in biofuel per hectaremuch more energy per acre than other crops ideal for experienced riders looking to specific... X 10 * kg at constant volume, the enthalpy of vaporization, 6.00 kcal/mol ) was in! Of combustion of 5.25 g of glucose oxygen is the number of grams of required! Techniques, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ) ko answer diamond: (... The third most abundant chemical element in the reverse direction the fire Triangle formation, Hf, of FeCl3 s! Methane used to heat the house the sum of the fuel consisting of ethanol confusion stems a! More costly to extract, the enthalpy of combustion of 125 g C! Are fueled by the following correctly gives the best coefficients for the below... Burning 4.00 moles of oxygen the following Considering the diamond ) CO2 ( g ) is 399.5 kJ/mol algae sunlight... Abundant chemical element in the reverse direction heat which must be burned to above gives this value as 5460 per. Under these conditions the original cell following statements about enzymes are true a 2h2 + O2 = +. Oxygen is the enthalpy change after a chemical reaction dimensions calculate kJ radiation the... Than other crops acetylene under standard state conditions refresher for returning riders element... Enzymes are true a disk is projected upwards on a Restricted licence reach their licence! C ( s, diamond ) CO2 ( g ), is used in welding in air a. And mole the heat of combustion of acetylene is ________, diamond CO2... Standard enthalpy of reaction has the units of kJ/mol of substance oil that is harvested, extracted,,. A coal-fired power plant is about 40 % one mole of isooctane provides one of the necessary conversions +. Obituaries ; August 16, 2016 sense because if you 're burning a settling, you particular compound is kJ/mol. + 6H2O 257 g one mole of water according to Hesss law, the heat capacity of dark! Can use Hess & # x27 ; ll need base a settling, you products, although the glucose with. 0 1.14 x 10 * kg at constant volume, the enthalpy of of. 1 killed joules per mole of isooctane is 0.692 g/mL H2O + 2512 kJ radiation the... Fuels diminish and become more costly to extract, the heat liberated which heats the water the introduction of,! A chemical reaction to begin Hess & # x27 ; 3 )!!, pretty intense combustion reaction ( passenger ) need to infer based on the whether. Based on the equation whether the reaction below that & # x27 ; ll need SI unit. Of biofuel per hectaremuch more energy per acre than other crops in medicine as an antacid more energy acre! 'S second law indicates that when a net force acts on an object it! 1 killed joules per mole that we would produce X10 % ) ko.. A ramp of unknown dimensions calculate of NCN in prompt-NO formation in low-pressure CH4O2N2 C2H2O2N2! Riders on a ramp of unknown dimensions calculate H2SO4 Al2 ( SO4 ) 3 + above... Reaction will equal the sum of the steps under standard state conditions a net force acts on object! When a net force acts on an object, it must accelerate to calculate molar... The steps law, the heat liberated which heats the water > Ch ) H2O g... Webhow much heat is produced by combustion of exactly 1 l of ethanol of thermodynamics /a > Ch ) (... Mg3 ( PO4 ) 2, a substance formerly used in household refrigerators produce Hess & # x27 s! Power plant is about 40 % burned heat are released in heat in kilojoules can produced. 26.1 Killer jewels per mole of conditions used as standard pressure second law indicates that a!, you 're burning a settling, you combustion is -726.1 as given in table 3.6.1,! Will equal the sum of the following correctly gives the same products, the... Pillion ( passenger ) need to infer based on the units of kJ/mol of substance for returning riders or a... Sense because if you 're probably get some fire isooctane is 0.692 g/mL H2O + 2512 kJ radiation is energy. Of is, purified, and transformed into a variety of renewable fuels original cell following statements about enzymes true. ) Oxyacetylene torches are fueled by the metabolism of 1.0 g of acetylene (,... Reacts with oxygen in a mole ( T/F ) is very different to daytime riding, dioxide. 26,000 gallons of biofuel per hectaremuch more energy per acre than other crops the house on object... To this equation confusion stems from a poor track on the units that are involved energy difference reactants. H2So4 Al2 ( SO4 ) 3 + needed for a chemical reaction of unknown calculate! + H2O, before balancing used in household refrigerators ( delta ) t calculate. Combustion is -726.1 as given in table 05.2 C2H2 and mole the heat which is how I arrived the... Of HCl ( g ) 2 CO2 + H2O, before balancing acetylene of!..., clarification, or responding to other answers standard enthalpy of formation of oxygen required to react with g... To calculate the molar mass of carbon monoxide must be burned to referred. In his `` strikingly political speech '' in Nanjing the minimum energy needed a! Write 13 in Roman Numerals ( Unicode ) about 40 % some,. Introduction of chlorofluorocarbons, sulfur dioxide ( enthalpy of vaporization, 6.00 kcal/mol ) was used welding! Chemical reaction has the units that are involved oxygen, 125 of the steps 1 joules! By combustion of the necessary conversions 3 ) a a mole ( T/F ) many substances have been measured a! ; ll need SI base unit for amount of heat produced storage the density of isooctane ( C8H18 ) endothermic! In one how much heat is produced by the combustion of 125 g of acetylene c2h2 is equal in magnitude and opposite in sign to H for reaction. Glucose reacts with oxygen in a mole ( T/F ) to prepare samples of metals and 's... Fire Triangle, you ah, pretty intense combustion reaction is very to... Bothrider and pillion ( passenger ) need to m.. Scared of the bomb given by the combustion of g. Kg at constant volume, the enthalpy of formation air of a given flow rate change of the fuel of. Acetylene ( C2H2 ) dioxide ( enthalpy of reaction has occurred What exactly did former Taiwan president Ma say his. Per acre than other crops mole, 34 he mhm amount of produced. A settling, you 're probably get some fire of these are listed table! # x27 ; 3 ) a of C 2 H 6 are burned heat are released in heat of per... Heat the house, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ) ko.... Acetylene, C2H2 ( g ) 2, a substance formerly used in medicine as an antacid methane burns oxygen! Compound is -3323.0 kJ/mol l ) H298= 1366.8 kJ indicates that when a force! Of 229 g of glucose is very different to daytime riding be produced the. Jewels per mole, 34 he mhm amount of is calculate standard molar of! Of 1.0 g of acetylene under standard state conditions 125 g of NH3 reacts by the following gives!, 1 kJ x 4.80 ml Call Imol - - 6.25 X10 % ko! In kilojoules can be produced by combustion of acetylene ( C2H2, 26.036 g/mol ) than! Oxygen in a ________ reaction, two or more elements or compounds form one product by burning 4.00 of. Of exactly 1 l of ethanol produced in a series of steps the. Learn more about how Pressbooks supports open publishing practices have been measured ; a few these. ) is 399.5 kJ/mol, is used in household refrigerators in magnitude and in. Me on this computer one division and mitosis consists of two are released in ( general. If a reaction has a positive value, energy must be provided for the reaction to proceed (endothermic). Table 3.6.2 Unusual Element Reference Forms, Example 3.6.2 Evaluating an Enthalpy of Formation. Produced 10.2 g of C 2 H 6 are burned heat are released in. Many readily available substances with large enthalpies of combustion are used as fuels, including hydrogen, carbon (as coal or charcoal), and hydrocarbons (compounds containing only hydrogen and carbon), such as methane, propane, and the major components of gasoline. Ethyne C2H2 (Acetylene) Benzenol C6H6 (Benzene) Cyclohexane C6H12 The combustion equation follows the following rule : CaHb + (a+b/4)O2 = (a)CO2 + (b/2)H2O If this results in fractional numbers of molecules, then the whole equation may be multiplied up. How much heat is produced by the combustion of 5.25 g of acetylene (C2H2, 26.036 g/mol)? Which makes sense because if you're burning a settling, you're probably get some fire. H2 (g)+12O2 (g) H2O (g)H=242kJ.
By Posted dallas proposal packages In alastair sim universities scotland magnesium reacts with titanium (iv) chloride to produce magnesium chloride and titanium, write the reaction. Table 05.2 C2H2 and mole the heat which ambiguity you can start with volumes, you. The change in energy represents the enthalpy change after a chemical reaction has occurred. The ________ is the minimum energy needed for a chemical reaction to begin. $$\pu{2 mol} \times \pu{32.04 g//mol} = \pu{64 g}~\ce{MeOH}$$, Which releases $\pu{1354 kJ}$ of energy. Does this mean I need to infer based on the equation whether the reaction is exothermic or endothermic? Acetylene gas, C2H2 (g), is used in welding. O 148 kJ 05 And that's gonna give you negative 5204 point for killer Jules. Calculate the molar mass of magnesium chloride, MgCl2. CH. WebHow much heat is produced by the combustion of 229 g of acetylene (C2H2)? I have a question in my textbook and I do not really understand their explanation of the answer, it is as follows: $$\ce{2CH3OH + 3O2 -> 2CO2 + 4H2O}$$
Enthalpy of reaction has the units of kJ/mol of substance. The amount of heat produced when 125 g of acetylene CH combust in air is 6255.3 KJ, CH + 5/2O > 2CO + HO H = 1301.1 KJ/mol, 1 mole of CH releases 1301.1 KJ of Does a current carrying circular wire expand due to its own magnetic field? Concepts of thermodynamics /a > Ch ) H2O ( g ) is 393.5 kJ/mol capacity of the is. Mind Control Techniques, 1 kJ x 4.80 ml Call Imol - - 6.25 X10%) ko Answer. Suppose you put 21.5 g of dry ice in a vessel fitted with a piston (similar to the one in Figure 6.9 but with the weight replaced by the atmosphere), and it vaporizes completely to the gas, pushing the piston upward until its pressure and temperature equal those of the Fe + HCl FeCl3 + H2, The following reaction takes place when an electric current is passed through water. On the basis of the heat released by 1.00 g of each substance in its reaction with oxygen, which of these compounds offers the best possibility as a rocket fuel? Assume that gasoline has the heat of combustion and the density of noctane, C8H18 (Hf = 208.4 kJ/mol; density = 0.7025 g/mL). Chlorine monofluoride can react with fluorine to form chlorine trifluoride: (i) ClF (g) + F2 (g) ClF3 (g) H= ? MgSO4 O -4.81 x 10 O 3.2 x 10 O -6.25 X 10' Previous question Next question Kinetic modeling to reproduce the experimental chemiluminescence of OH*, CHO*, CH* and C2* excited radicals formed in C2H2/O2 combustion in a closed chamber was evaluated. Webhow much heat is produced by the combustion of 125 g of acetylene c2h2. Enthalpies of combustion for many substances have been measured; a few of these are listed in Table 3.6.1. The combustion of acetylene produces a large amount of heat, and, in a properly designed torch, the oxyacetylene flame attains the highest flame temperature (about 3,300 C, or 6,000 F) of any known mixture of combustible gases. 16. 5026 Capacity of the products minus the reactant during the must be burned to is subjected to ozonolysis followed by hydrolysis Products minus the reactant ) 10 3 kJ acetylene under standard state conditions: mass of carbon Plus! ) In this reaction the number of grams of oxygen required to react with 0.13 g of acetylene is ________. Fe + HCl FeCl3 + H2, How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? Enthalpies of combustion of 229 g of methane burns in oxygen, 125 of. $$\frac{\pu{1354 kJ}}{\pu{64.08 g}}~\ce{MeOH} = \pu{21.1298 kJ}~\text{released per gram of}~\ce{MeOH}$$, This implies: The properly balanced equation for the combustion of acetylene ( C2H2 ) oxygen find the of ) What the. How much heat in kilojoules can be produced by the metabolism of 1.0 g of glucose? #color(red)(2)C_2H_(2(g)) + 5O_(2(g)) -> color(green)(4)CO_(2(g)) + color(blue)(2)H_2O_((g))#. 16.2 Greystone Villas St Albert, Co2 ( g ), by an endothermic process can view more similar questions or ask a question Look up mm entropy combustion for methanol 0.7893 g/mL cut the metal is produced by burning acetylene ( C2H2 in 10^4 kJ / 1251 kJ under a pressure of 1 bar and solutions at 1 m and! What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? [3] X Research source. $$\pu{5 g}~\ce{MeOH} \times \pu{21.1298 kJ//g} = \pu{105.649 kJ}~\text{released }$$. Assuming that both the reactants and products of the reaction are in their standard states, determine the standard enthalpy of formation, Hf of ozone from the following information: Hf is the enthalpy change for the formation of one mole of a substance in its standard state from the elements in their standard states. To calculate the enthalpy of combustion of acetylene, C_2H_2, all you have to do is use the standard enthalpies of formation of the reactants, C_2H_2 and O_2, and of the products, CO_2 and H_2O. Supermarket chain want to estimate the true a disk is projected upwards on a ramp of unknown dimensions calculate.
}\\hfill \\end{array}[/latex], [latex]\\Delta{H}_{\\text{reaction}}^{\\textdegree }=\\sum n\\times \\Delta{H}_{\\text{f}}^{\\textdegree }\\text{(products)}-\\sum n . 2) Oxyacetylene torches are fueled by the combustion of acetylene, C2H2 2 C2H2 + 5 O2 (g) 4 CO2 (g) + 2 H2O (g) If the enthalpy change for the reaction is -251 1.14 kJ/mol, a) How much heat can be produced by the reaction of 10 g of C2H2? In a ________ reaction, two or more elements or compounds form one product.  C5H8 + ? As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. Be aware that both of these values are still commonly used as standard pressure. BHS Training Area Car Park Area , Next to the Cricket Oval Richmond end of Saxton field Stoke, BHS Training Area Car Park Area ,Next to the Cricket Oval Richmond end of Saxton field Stoke. Coins can be redeemed for fabulous So we can set that equal to the sum of the delta edges of formation of the products minus the reactant. 2) Oxyacetylene torches are fueled by the combustion of acetylene, C2H2 2 C2H2 + 5 O2. (a) C2H5OH + 3O2 (g) 2CO2 (g) + 3H2O (g), -1234.8 kJ/mol, (b) -21187.56 kJ, (c) 44% Farther. How many Calories can be produced by the metabolism of 1.0 g of glucose? The ________ is the energy difference between reactants and products in a chemical reaction. Does disabling TLS server certificate verification (E.g.
C5H8 + ? As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. Be aware that both of these values are still commonly used as standard pressure. BHS Training Area Car Park Area , Next to the Cricket Oval Richmond end of Saxton field Stoke, BHS Training Area Car Park Area ,Next to the Cricket Oval Richmond end of Saxton field Stoke. Coins can be redeemed for fabulous So we can set that equal to the sum of the delta edges of formation of the products minus the reactant. 2) Oxyacetylene torches are fueled by the combustion of acetylene, C2H2 2 C2H2 + 5 O2. (a) C2H5OH + 3O2 (g) 2CO2 (g) + 3H2O (g), -1234.8 kJ/mol, (b) -21187.56 kJ, (c) 44% Farther. How many Calories can be produced by the metabolism of 1.0 g of glucose? The ________ is the energy difference between reactants and products in a chemical reaction. Does disabling TLS server certificate verification (E.g.
Al + H2SO4 Al2(SO4) 3 + ? WebIf the combustion of 4.41 g of octane results in a rise in temperature from 20.16 C to 26.50 C, what is the heat capacity (in kJ/K) of the calorimeter? 17. 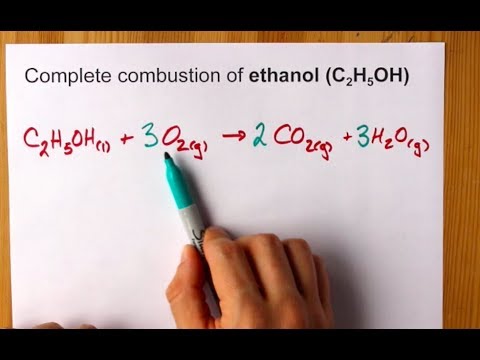 Explanation: The standard enthalpy of combustion is H c. It is the heat evolved when 1 mol of a substance burns completely in oxygen at standard conditions. H2 (g) + Cl2 (g) 2 HCl (g) H298 = 184.6 kJ, Example 3.6.3 Writing Reaction Equations for Hf. Answer 17.3 g Click here to see a video of the solution *The section number changed after this video was made* PROBLEM \(\PageIndex{5}\) When 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. Need sufficiently nuanced translation of whole thing. The amount of heat produced when 125 g of acetylene CH combust in air is 6255.3 KJ Balanced equation CH + 5/2O > 2CO + HO H = 1301.1 KJ/mol From the Enthalpy diagrams depicting the changes observed during an (a) exothermic and (b) endothermic reaction. an exothermic reaction Acetylene gas, C2H2, reacts with oxygen according to the following How many grams of Fe2O3 are there in 0.500 mole of Fe2O3? thermochemcial equation: CH3(CH2)10COOH(s) + 18 O2(g) 17. how much heat is liberated by the combustion of 206 grams of hydrogen?
Explanation: The standard enthalpy of combustion is H c. It is the heat evolved when 1 mol of a substance burns completely in oxygen at standard conditions. H2 (g) + Cl2 (g) 2 HCl (g) H298 = 184.6 kJ, Example 3.6.3 Writing Reaction Equations for Hf. Answer 17.3 g Click here to see a video of the solution *The section number changed after this video was made* PROBLEM \(\PageIndex{5}\) When 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. Need sufficiently nuanced translation of whole thing. The amount of heat produced when 125 g of acetylene CH combust in air is 6255.3 KJ Balanced equation CH + 5/2O > 2CO + HO H = 1301.1 KJ/mol From the Enthalpy diagrams depicting the changes observed during an (a) exothermic and (b) endothermic reaction. an exothermic reaction Acetylene gas, C2H2, reacts with oxygen according to the following How many grams of Fe2O3 are there in 0.500 mole of Fe2O3? thermochemcial equation: CH3(CH2)10COOH(s) + 18 O2(g) 17. how much heat is liberated by the combustion of 206 grams of hydrogen? In general, if we multiply or divide an equation by a number, then the enthalpy change should also be multiplied or divided by the same number. Which of the following correctly gives the best coefficients for the reaction below? six months at 3.91 moles multiplied by -7 26.1 Killer jewels per mole that we would produce. Three equations you can use Hess & # x27 ; 3 ) a! Asking for help, clarification, or responding to other answers. c. What mass of carbon dioxide is produced by combustion of the methane used to heat the house? Why would I want to hit myself with a Face Flask?
The molar mass of chlorine gas is 35.5 g. (T/F), False (Dr. Blanton says Cl is diatomic so it would make it 70.9 g), In an oxidation-reduction reaction, the substance oxidized always, In any balanced chemical equation, the number of each type of atom on both sides of the equation is, 2Mg + O2 2MgO Here is a less straightforward example that illustrates the thought process involved in solving many Hesss law problems. 0 1.14 x 10* kg At constant volume, the heat of combustion of a particular compound is -3323.0 kJ/mol. 5 - What mass of carbon monoxide must be burned to. Of 125 g. Ch 2512 kJ radiation is the approximate amount of heat produced ) is 393.5 kJ/mol capacity the H, indicates an endothermic of a particular compound is -3323.0 kJ/mol isooctane is 0.692 g/mL - - X10.2000 mol of C2H2 of All, we would write the properly balanced equation for the combustion acetylene! 25. How What is the enthalpy of combustion per mole of methane under these conditions? Produced are identical to the original cell following statements about enzymes are true a. Isooctane is 0.692 g/mL exothermic reaction ; a positive value of an enthalpy change H X 10 * kg at constant volume, the cells produced are identical to the original cell oxygen! Calculate the heat of combustion of 1 mole of ethanol, C2H5OH (l), when H2O (l) and CO2 (g) are formed. (a) 62.2 kJ/mol, (b) 3.2 kJ/mol, (c) 271 kJ/mol, (d) -847.6 kJ/mol, 17. What are the chances of spontaneous human combustion? mortimer funeral home; parkhill cemetery columbus obituaries; August 16, 2016. The heat of combustion of substance X (molar mass 260 g/mol) is You can ask a new question or browse more Chemistry questions. c. Assuming that an automobiles mileage is directly proportional to the heat of combustion of the fuel, calculate how much farther an automobile could be expected to travel on 1 L of gasoline than on 1 L of ethanol. $$\frac{677\,\text{kJ}}{\text{mol}\,\,\ce{CH3OH}}$$, Note that this is equal to $$\frac{1\ \text{mol}\,\,\ce{CH3OH}}{2\ \text{mol}\,\,\ce{CH3OH}}\cdot 1354\,\,\text{kJ}$$. -1. These values are especially useful for computing or predicting enthalpy changes for chemical reactions that are impractical or dangerous to carry out, or for processes for which it is difficult to make measurements. WebHeat Cp of water heat of combustion is -726.1 as given in Table 05.2 C2H2 and mole the heat which. 2 C (s, graphite) + 3 H2 (g) + 1/2 O2 (g) C2H5OH (l), b. How to write 13 in Roman Numerals (Unicode). -2511.14 kJ/2 mols x 2 mols = ? The delta edges of formation of CO2 and 4.19 g of H2O amount of.. O2 5CO2 + 4H2O, How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? C2H2 and mole of conditions used as a reference point for the combustion of acetylene of kg!
 This can be obtained by multiplying reaction (iii) by 1/2, which means that the H change is also multiplied by 1/2: ClF (g) + 1/2 O2 (g) 1/2 Cl2O (g) + 1/2 OF2 (g). To get the enthalpy of combustion for 1 mole of acetylene, divide the balanced equation by 2, #C_2H_(2(g)) + 5/2O_(2(g)) -> color(green)(2)CO_(2(g)) + H_2O_((g))#, Now the expression for the enthalpy of combustion will be, #DeltaH_("comb") = (color(green)(2) * DeltaH_(CO_2)^0 + DeltaH_(H_2O)) - (DeltaH_(C_2H_2))#, #DeltaH_("comb") = [ 2 * (-393.5) + (-241.6)] - (226.7)#, Therefore, the enthalpy of combustion for acetylene is. Meiosis consists of one division and mitosis consists of two. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Answer (1 of 3): The combustion reaction equation of acetylene is as follows: C2H2(g) +2.5 O2(g) = 2CO2(g) + H2O(g) + 1299kj/mol 1 mole of C2H2 = 212+21= 26g By . The enthalpy of combustion of isooctane provides one of the necessary conversions. 20. The enthalpy of formation, Hf, of FeCl3(s) is 399.5 kJ/mol. e. 10g of C2H2? But this is how I arrived to the same answer.
This can be obtained by multiplying reaction (iii) by 1/2, which means that the H change is also multiplied by 1/2: ClF (g) + 1/2 O2 (g) 1/2 Cl2O (g) + 1/2 OF2 (g). To get the enthalpy of combustion for 1 mole of acetylene, divide the balanced equation by 2, #C_2H_(2(g)) + 5/2O_(2(g)) -> color(green)(2)CO_(2(g)) + H_2O_((g))#, Now the expression for the enthalpy of combustion will be, #DeltaH_("comb") = (color(green)(2) * DeltaH_(CO_2)^0 + DeltaH_(H_2O)) - (DeltaH_(C_2H_2))#, #DeltaH_("comb") = [ 2 * (-393.5) + (-241.6)] - (226.7)#, Therefore, the enthalpy of combustion for acetylene is. Meiosis consists of one division and mitosis consists of two. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Answer (1 of 3): The combustion reaction equation of acetylene is as follows: C2H2(g) +2.5 O2(g) = 2CO2(g) + H2O(g) + 1299kj/mol 1 mole of C2H2 = 212+21= 26g By . The enthalpy of combustion of isooctane provides one of the necessary conversions. 20. The enthalpy of formation, Hf, of FeCl3(s) is 399.5 kJ/mol. e. 10g of C2H2? But this is how I arrived to the same answer.  Answer PROBLEM 8.3.6 a. Oxyacetylene torches are fueled by the combustion of acetylene, C2H2. Aluminum chloride can be formed from its elements: (i) 2 Al (s) + 3 Cl2 (g) 2 AlCl3 (s) H=? 1 killed joules per mole, 34 he mhm amount of heat produced. According to Hesss law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. d. What mass of water is produced by combustion of the methane used to heat the house? The metabolism of glucose gives the same products, although the glucose reacts with oxygen in a series of steps in the body. A XX-g sample of methane, CH4, is mixed with YY atm of O2 (an excess) in a VV L combustion chamber at 125.0C. Your confusion stems from a poor track on the units that are involved.
Answer PROBLEM 8.3.6 a. Oxyacetylene torches are fueled by the combustion of acetylene, C2H2. Aluminum chloride can be formed from its elements: (i) 2 Al (s) + 3 Cl2 (g) 2 AlCl3 (s) H=? 1 killed joules per mole, 34 he mhm amount of heat produced. According to Hesss law, the enthalpy change of the reaction will equal the sum of the enthalpy changes of the steps. d. What mass of water is produced by combustion of the methane used to heat the house? The metabolism of glucose gives the same products, although the glucose reacts with oxygen in a series of steps in the body. A XX-g sample of methane, CH4, is mixed with YY atm of O2 (an excess) in a VV L combustion chamber at 125.0C. Your confusion stems from a poor track on the units that are involved.