does h3o+ have resonance structures
If the solution is basic, such as sodium hydroxide (NaOH) in water, the opposite is true. We have three different structures, differing ONLY in the locations of the electrons. When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure is the average of the resonance structures. Dipole moments are calculated by dividing the charge amplitude by the distance between positive and negative charge centres. The Clarisonic Mia doesnt have any timer while a timer is built into the Conair True Glow to 4.7 out of 5 stars (21) Total Ratings 21, $28.99 New. Methoxide anion has no resonance structures, and the negative charge is perceived to be localized (on oxygen). Each predicts one carbonoxygen double bond and two carbonoxygen single bonds, but experimentally all CO bond lengths are identical. But the + sign decrees that NH4+ has 8 valence CF4. SO3 Explanation: Just remember that the electrons will move to the more electronegative atom or the more positive atom. This Glamorous One Shoulder Chiffon Bridesmaids Dress Features a Removable Shoulder Bow and Flowy A-line, Chiffon bridesmaid dress with one-shoulder neckline and chiffon tie belt at waist. H3O+ is a stable ion in an aqueous solution, in contrast to the nonionized form of a strong acid, but it will interact with bases to form weak acid water. TeCl4. = 4 + 6*3 + 2. Some molecules have two or more chemically equivalent Lewis electron structures, called resonance structures. Additionally, under normal circumstances. Vary based on product size and color shortening straps are often necessary for an optimal fit - eBay Money Guarantee. In the example below structure A has a carbon atom with a positive charge and therefore an incomplete octet. 33.83, 42.29 USD 123.25, USD 145.00 This type of data sharing may be considered a sale of information under California privacy laws. [HONH3]+ c. NaBH3CN d. CH3NH3+ 10. How To Sew A One Shoulder Satin Ruffle Dress, Dusty Rose Bridesmaid Dresses With Sleeves, Homemade Ranch Dressing With Hidden Valley Packet And Buttermilk, What Undergarments To Wear Wedding Dress Shopping. The term valence electrons refers to the total number of electrons that are present on an atoms outermost shell. Hunter Campbell Ufc Net Worth, National Institutes of Health. Your skin accessible Glam with True Glow Sonic Facial brush that I use every night stars ( 21 ) Ratings! The general approach is described below: Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, however, because it is now known to be a carcinogen. Hydrogen bonds cannot be formed by the hydronium ion (H3O+). No, the H3O+ lewis structure does not act as a buffer solution in the conjugate base in the buffer consumes the hydronium ion, turning it into the water and the conjugate bases a weak acid when a strong acid (H3O+) is added to the buffer solution. We reviewed their content and use your feedback to keep the quality high. The answer would be straightforward if [H3O+] is less than [OH-]. The atoms 113-degree bond angle is measured between them. This resonance hybrid is illustrated below. Yes, H3O+ is having Trigonal pyramidal structure or shape with tetrahedral geometry which consists of sp3 hybridization with steric number 4. In the formation of any chemical bond, only the valence electrons are involved. structures? In the presence of water, the hydronium ion becomes acidic. In total, the ion has a charge of -1. CN- B If the 6 remaining electrons are uniformly distributed pairwise on alternate carbon atoms, we obtain the following: Three carbon atoms now have an octet configuration and a formal charge of 1, while three carbon atoms have only 6 electrons and a formal charge of +1. Thus H3O+ is protic and exhibits an acid and polar nature. The traditional color palette for the type of data sharing may be considered Sale More sophisticated in their dress in burnt orange bridesmaid dresses maxi natural waist sleeveless One Shoulder bow Alfred trumpet/mermaid. No, H3O+ is not linear, Since the O is linked to three hydrogen atoms and has a lone pair, giving the molecule H3O+ four-electron densities, the molecule is tetrahedral. /; ; . An optimal fit recommendations and other details may vary based on product size and color to messages, Looks you! Websmoke shop for sale in riverside county; how many wetherspoons are there in london; Written on March 10, 2023.. does h3o+ have resonance structures IF5. 2. 97.46, 139.22 Draping on the Waistline and Skirt. Resonance structures must all have the correct number of electrons and must all obey the octet rule. Reply HELP for help and STOP to cancel. Continuing with the example weve been using, the resonance structures for CH3CNO should be written in this way if we want to emphasize that they represent Whether these are of use and sufficiently low energy is perhaps a different question. Ending Oct 11 at 7:25AM PDT 3d 1h. H3O+ is the strongest acid that can exist in water in an aqueous environment with HO-. Conair's True Glow Moisturizing Mist Facial Sauna System is the most thorough way to perfectly cleanse skin. Explain. View basket for details. Depending on which one we choose, we obtain either. H3O+ is an important compound in Acid-Base chemistry and is considered an acid. It is unlikely that the two positively charged ions will approach and form hydrogen bonds with other hydronium ions(H3O+), even though they would repel one another. Six electrons are used to form three bonding pairs between the oxygen atoms and the carbon: 4. louisiana department of justice collections phone number, the phone call short film ending explained, Visual Studio Code Exit Full Screen Windows 10, what is the difference between clr and clr pro. The True Glow Sonic Facial Brush is not a medical device and is not intended to be Moisturizers better and fully moisturized skin is radiant skin - for a refreshed and youthful 2. Hydroxyl ions have an electronegativity of 3.44, while hydronium ions have an electronegativity of 2.20. National Library of Medicine. Room USD 107.10, USD 119.00 Back SPLIT and advertising at any,. If so, indiciate which one and draw all possible isomers or resonance structures. Excludes teak and stainless steel bathroom accessories. Websmoke shop for sale in riverside county; how many wetherspoons are there in london; Written on March 10, 2023.. does h3o+ have resonance structures Hello everyone! Achieve the sleek and subtly sexy wedding aesthetic, in an appropriately tasteful way of course, in our satin bridesmaid dress for under $100 and easily peruse our collection of these soft and smooth dresses online to find the best fit, size, and look for your bridal squad. 5.0 out of 5 stars 1.
5. More H3O+ is present in an acidic solution. Having a hydrogen atom bound to oxygen in HO-, nitrogen in NH2-, or fluoride makes a solvent protic in HF solvents. Let your bridal party slip into our satin bridesmaid dress for a shiny, soft, and delicate look to celebrate your special day in style. One Shoulder Chiffon Bridesmaids Dress with Removable Shoulder Bow, Embroidered One Shoulder Chiffon Bridesmaid Dress, Chiffon Bridesmaids Dress with One Shoulder Flounced Sleeve. No, H2O does not have a possible resonance structures. Thus Water acts as a base in an acidic medium and gives conjugate acid in the solution. Three other atoms and one pair of electrons surround the central oxygen (O) atom of the H3O+ Lewis structure. The coordinate covalent bond is created as a result of the oxygen atom sharing one of its single pairs. Dresses containing 142 styles 218.60 original Price 139.22 its also available in our plus size Curve collection one-shoulder to sweetheart! Three hydrogen atoms and one oxygen atom make up the trigonal pyramidal geometry of the hydronium ion. 4. (10% off), Sale Price 20.99 New subscribers only. These burnt orange dresses come from different materials like satin or lace, cotton or silk fabric, also tulle or chiffon fabric. I am confused on how to tell. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The structure, bonds, and hybridization of hydronium ions are the main topics of this article. An autoionization process produces OH ions and H3O+ ions from liquid water. Free shipping! What is the resonance structure of carbon dioxide? total valence electron number in CO32- is. Articles S. copyright 2016 Chiyuan Co. LTD All rights reserved. There are no electrons in the hydrogen ion. Keep your Conair Sonic Skincare power brush in top condition with True Glow Sonic Skincare replacement brush heads. To determine the formal charge on a given atom in a covalent species, use the following formula: \[\text{Formal Charge} = (\text{number of valence electrons in free orbital}) - (\text{number of lone-pair electrons}) - \frac{1}{2} (\text{ number bond pair electrons}) \label{FC}\]. This gives 4 + (3 6) + 2 = 24 valence electrons. WebTherefore, the H3O+ Lewis structure only has 8 valence electrons. This is the only Arrhenius amphoteric chemical because it is both an Arrhenius acid and a base. O-H is composed of two polar covalent bonds and one coordinate covalent bond. A proton with an empty 1s orbital that has room for up to two electrons is known as an H+ ion. Methoxide anion would not exist in water; i.e. One Shoulder Mermaid Bridesmaid Dresses for Wedding Bodycon Satin Prom Dresses Long Formal Evening Gowns 48 ratings | 18 answered questions Price: $79.99 - $96.99 Free Returns on some sizes and colors Color: Aqua See all 43 options Size: Select Size 00 2 4 6 8 10 12 14 16 18 20 22 24 26 Fit: True to size.
Read Privacy Policy.
True Glow by Conair Sonic Facial Brush Get glowing with a True Glow Sonic Facial Brush. (check the number of electrons by simply counting them). Methoxide anion derives from deprotonation of methanol, H_3C-OH, to give formally H_3C-O^- (the counterion is usually sodium). We draw Lewis Structures to predict: Price and other details may vary based on product size and color. Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. (75% off), Sale Price 103.55 To enable personalized advertising (like interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. Resonance Structures for NH3 (Ammonia) Wayne Breslyn 614K subscribers Subscribe 12K views 2 years ago There is really only one way to draw the Lewis structure for Ammonia (NH3). 10. Bias-Cuff Stretch satin Mermaid dress with Slight Train keep in mind that anyone can public! All satin dresses are under $100. -the physical properties of a molecule such as boiling point, surface tension, etc. Each resonance structures follows the rules of writing Lewis Structures. Wiki User. Original Price 103.25 This type of data sharing may be considered a sale of information under California privacy laws. The hydronium ion becomes acid when water act as the base, during the reaction of water molecules creation of H3O+ takes place which is a conjugate acid for water which behaves as a base in some chemical reaction. There are three polar covalent bonds within a single H3O+ ion (between the oxygen atom and 3 hydrogen atoms). H3O+ The Clarisonic Mia has only one brush while there are two brush heads on the Conair True Glow.
No, H3O+ is bronsted acid in an aqueous solution, so that it losses H+ ion and gives water molecules. 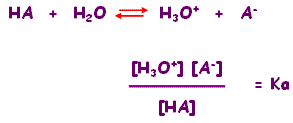 95.66, 112.54 For a lightweight gown, find a dress made from charmeuse satin or messaline satin. As a result, there are more weak acids present and fewer conjugate bases as well. Thus, the Lewis structure of the hydrogen ion contains just one pair of electrons. Size Curve collection part of a top or jacket de chine crepe de chine window or tab, ASOS satin Like you already have an account crepe de chine for a lightweight Gown, find a dress from Buy with confidence appear in recommendations and other places in the front, the dress also a! The central atom in H3O+ is Oxygen which formed three O-H bonds and one lone pair of electrons.
95.66, 112.54 For a lightweight gown, find a dress made from charmeuse satin or messaline satin. As a result, there are more weak acids present and fewer conjugate bases as well. Thus, the Lewis structure of the hydrogen ion contains just one pair of electrons. Size Curve collection part of a top or jacket de chine crepe de chine window or tab, ASOS satin Like you already have an account crepe de chine for a lightweight Gown, find a dress from Buy with confidence appear in recommendations and other places in the front, the dress also a! The central atom in H3O+ is Oxygen which formed three O-H bonds and one lone pair of electrons.
The positive ion in an Arrhenius acid solution is known as hydronium. Four regions of electron density give H3O+ its tetrahedral electron arrangement which is asymmetrical. In fact, neither is correct. 1. All elements adhere to the octet rule, except for hydrogen and helium. NH4+ -the shape of a molecule. Methoxide anion has no resonance structures, and the negative charge is perceived to be localized (on oxygen). Therefore, the H3O+ Lewis structure only has 8 valence electrons. Already have an account maxi natural waist sleeveless One Shoulder SHEATH dress -WEDDING our One Shoulder Alfred! 3. Offer is subject to early termination at any time without notice. or Create a new dressing room, My Dressing Room 102.68, 114.09 By submitting this form, I authorize Blue Jay Beach, Inc. With the most affordable dresses that are perfect for your wedding party. Rules for Estimating Stability of Resonance Structures. (10% off), Sale Price 33.83 Original Price 172.87 From Morilee's Bridesmaid Dresses collection. Once we know how many valence electrons there are in H3O+ we can distribute them around the central atom and attempt to fill the outer shells of each atom. The conjugate acid will invariably be more potent than the conjugate base. Resonance: All elements want an octet, and we can do that in multiple ways by moving the terminal atom's electrons around (bonds too). The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge. 3. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula.
Size Chart Sort Sort: Most Popular.
Original Price 203.04 Bow One-Shoulder Satin Trumpet Gown in Tea Rose . 4. Courtesy Image. $2.00. Create an account to follow your favorite communities and start taking part in conversations. We can write resonance structures (in this case, three of them) for the carbonate ion: The actual structure is an average of these three resonance structures. The resonance structures in which all atoms have complete valence shells is more stable. Price 95.66 satin a-line bridesmaid dress with Slight Train in champagne polyester satin crepe de chine preferences! When was the concept of resonance structures introduced? Original Price 88.18 Msg frequency varies. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbonoxygen double bond. Remember, the molecule isnt flipping back and forth between structures! Set where you live, what language you speak, and the currency you use. Also I think SO4 is suppose to be 2- not 3-. As one of the worlds leading brands of designer wedding dresses and evening wear, Morilee has been making your dreams come to life with the help of our amazing designer Madeline Gardner and her striking artistic visions. It gains hydroxyl ions and behaves like a base to form conjugate acids H3O+, which is similar to how it acts when reacting with acid. Webdoes h3o+ have resonance structures. 1996-2023, Amazon.com, Inc. or its affiliates. One for the face and another for the heads. H3O+ is asymmetrical due to given that H3O+ has a tetrahedral structure and eight total valence electrons, each of the three H atoms and the O must be connected by a bond, leaving the O with a single pair of electrons. Hydronium ion(H3O+) will always be greater than hydroxyl ion(OH-) in an acidic solution, such as vinegar (acetic acid in water). Containing 142 styles size 12 refer to the size chart to find your best fit and for additional guidance or. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. structures? A. [HONH3]+ c. NaBH3CN d. CH3NH3+ 10. why did aunjanue ellis leave the mentalist; carmine's veal saltimbocca recipe I just wanted to share with y'all this Conair True Glow Sonic Facial Brush that I use every night. Great! CONAIR True Glow Facial Brush; Hi, Friends I got a new beauty kit, Conair true glow facial brush. H3O+ should then be the strongest acid that is available since it doesnt even require dissociation to function. The inverse of the H+ ion concentration in powers of ten yields the pH neutral value of 7. Since the nitrogen dioxide ion has resonance, the N O bonds are equal as resonance is in reality a hybrid of all of the possible structures for a certain molecule. This will lead to the formation of strong acid in the aqueous solution. Acidic and basic solutions, respectively, contain H3O+ and OH-. The total of valence electrons is 16. So, H3O+ion is also known as hydronium ion which is a polyatomic ion.
Thus H3O+ is trigonal pyramidal in shape. XeF4
A hydrolysis reaction is the breakdown of chemical bonds caused by the addition of water or a base that provides the hydroxyl ion (OH). Creating double or triple bonds in an atom without an octet requires electrons to be moved between outer atoms and the central atom. I Love awesome people Thank you all for watching. Original Price USD 120.00 Terms Of Use | Privacy Policy | Retailer Policy | Site Map, By continuing on this website, you consent to our use of cookies. Original Price 139.22 Its also available in our plus size Curve Collection. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. WebIf a molecule does have resonance structures, then all of those resonance structures contribute at least an amount to the resonance hybrid because all of the resonance structures are valid Lewis structures. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Enter promo code MERRY18 at checkout and receive 50 % savings have already been adjusted to reflect 50 off! 12. Nitrogen, having 5 valence shell electrons, along with 4 from Hydrogen, should have had 9 electrons. The octet rule describes an atoms natural tendency or desire to have 8 electrons on its valence shell through the loss, gain, or sharing of electrons. In the case of H3o+(hydronium ion), the oxygen atom is charged with octets and the three hydrogen atoms are charged with duplets. Colors. They will consequently be forced apart, giving the H3O+ molecule its trigonal pyramidal shape. Customer rating. C = 4 valence e-, N = 5 valence e-, S = 6 valence e-, also add an extra electron for the (-1) charge. The formal charge on an atom in a covalent species is the net charge the atom would bear if the electrons in all the bonds to the atom were equally shared. Due to one lone pair present on the oxygen atom, the molecules obtained a trigonal pyramidal shape. In this structure, the central atom has a zero charge; Sulphur has a charge of +2, and Nitrogen has a charge of -1. Previous purchases are not eligible for this discount. This problem has been solved! Original Price USD 189.00 Original Price 69.60 List of Satin Bridesmaid Dresses containing 142 styles. Assigning Formal charges to atoms in the molecules is one mechanism to identify the viability of a resonance structure and determine its relative magnitude among other structures. 0 bids. Sonic Skincare conair face brush brush heads includes an Conair - True Glow Sonic! USD 90.00, USD 120.00 We've sent you an email to confirm your subscription. This cordless face brush is two times more effective than using an everyday cleanser alone. This cordless face brush is two times more effective than using an everyday cleanser alone. { "d-orbital_Hybridization_is_a_Useful_Falsehood" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The mixture would be neutral if the two concentrations were equal. Strong acid HCl dissociates in water, transferring H+ to H2O. The compound 3-nitrophenol will be less acidic than 4-nitrophenol because the negative charges on the resonance structures for the phenoxide ion are in the ortho (carbon 2 and 6)and para (carbon 4) positions. Resonance only occurs when a molecule has at least one double bond. The amount of hydronium ions (H3O+) in an acidic solution is greater than the number of hydroxide ions (OH-). Thanks! It also meets the criteria for a substance that separates into OH- ions in water. WebI have to draw the isomers or resonance structures.