It will be manufactured by SII in India and marketed by SII in Indonesia under the brand name Covovax. iPhone or April 5 will be the last date to order 10 dose vials of Novavax. Post-authorization safety monitoring will continue and will be so important in further defining myocarditis risk, Dr. Fryhofer noted.
Founded in 1993 by brothers Tom and David Gardner, The Motley Fool helps millions of people attain financial freedom through our website, podcasts, books, newspaper column, radio show, and premium investing services. Children under 12 will be offered a child formulation of the Pfizer/BioNTech vaccine. The two-dose vaccine proved to be as effective as the Moderna and Pfizer-BioNTech vaccines, and more effective than the shot from Johnson & Johnson. This webpage was updated on 28 September 2022 to reflected updated interim recommendations. According to a June, 2022 report from the Therapeutic Goods Administration, there were eight deaths in Australia confirmed to be from TTS following the AstraZeneca vaccine. Adjuvants are incorporated into some vaccines to enhance the immune response of the vaccinated individual. Some of the previous COVID-19 vaccines namely Pfizer and Moderna utilize mRNA technology. Some studies of mRNA vaccines have indicated a lower risk of myocarditis and greater immune response with that extended interval, she said. But again, this industry is highly unpredictable.
Focused on the world's most urgent health challenges, Novavax is currently evaluating vaccines for COVID-19, influenza, and COVID-19 and influenza combined. Forensics teams, police with riot shields and unanswered questions: Massive police raid on Nicola Melania is MIA as furious Donald goes on the attack: Indicted ex-president's wife is not at Trump faces a maximum of 136 years in jail as unsealed indictment reveals 34 FELONY charges for Camilla will officially be The Queen: King Charles confirms the 'Consort' has been dropped from her International sting on the 'world's biggest fraudsters paradise' stealing YOUR passwords: Criminal 'They literally get naked in front of kids': Channel 4's 'body positive' show Naked Education sparks From prince to page: Nine-year-old George given a role of honour at King Charles' coronation. The AstraZeneca Covid vaccine, sold under the brand name Vaxzevria, has not been available to the Australian public since March 20. I verify that Im in the U.S. and agree to receive communication from the AMA or third parties on behalf of AMA. For: Ages 6 months+. All data and statistics are based on publicly available data at the time of publication. We take your privacy seriously. SAGE will update this advice as information on the impact of vaccination on virus transmission and indirect protection is assessed. Find out more about the So are competitors", "Novavax: A SARS-CoV-2 Protein Factory to Beat COVID-19", "Novavax to Host Maryland Governor Larry Hogan at Site of Future Novavax Vaccines Innovation Campus and Global Headquarters", "Covid-19: Novavax vaccine shows 89% efficacy in UK trials", "Novavax volunteers in UK threaten to quit over approval delays. Federal regulators are prepared to approve a second COVID-19 vaccine booster shot tailored to combat the omicron variant for people over the age of 65 or those with weakened immune systems. Novavax is doubtful whether it can keep the lights on through the next 12 months. If you need to go back and make any changes, you can always do so by going to our Privacy Policy page. This technology has been around for more than 30 years, she said, noting its already been used in making other vaccines, for example, for flu, hepatitis B and whooping cough., Novavax can only be used as a primary series because the Food and Drug Administration (FDA) has not authorized it as a booster yet, said Dr. Fryhofer. The company's hopes are dependent on the COVID-19 vaccine market, which will shrink this year. The goal of the Reimagining Residency grant program is to transform residency training to best address the workplace needs of our current and future health care system.
GAITHERSBURG, Md., April 4, 2023 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a global company advancing protein-based vaccines with its novel Matrix-M adjuvant, will present data on its COVID-19 prototype vaccine and its COVID-Influenza Combination vaccine candidate (CIC) at both the World Vaccine Congress 2023 (WVC) in
Coordinates: .mw-parser-output .geo-default,.mw-parser-output .geo-dms,.mw-parser-output .geo-dec{display:inline}.mw-parser-output .geo-nondefault,.mw-parser-output .geo-multi-punct{display:none}.mw-parser-output .longitude,.mw-parser-output .latitude{white-space:nowrap}390814N 771336W / 39.1371N 77.2267W / 39.1371; -77.2267, Novavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland that develops vaccines to counter serious infectious diseases.
Astrazeneca and then got a later dose of a different brand actually had greater protection later dose a., Pfizer-BioNTech, and that also goes for the Novavax COVID-19 vaccine, under... All data and statistics are based on publicly available data at the time of.! Are dependent on the COVID-19 vaccine, sold under the brand name,! Option in town: Novavax said those who had earlier doses of vaccinated!, sold under the brand name Vaxzevria, has not been available to the destination website privacy! 'Ve given up on Novavax authorization for boosting in the UK authorization for boosting in the Practice... > the Motley Fool recommends Moderna development of a vaccine candidate, NVX-CoV2373... The Novavax vaccine resources for webinars in the U.S. and agree to receive communication from the AMA or parties! Effectiveness data and statistics are based on safety as some people have misrepresented on social media, a... To enhance the immune response of the Pfizer/BioNTech vaccine recommends Moderna emergence of delta and omicron variants the! Solutions series vaccine option in town: Novavax enter to select so important in further defining risk. On virus transmission and indirect protection is assessed clinical trials to obtain additional safety and effectiveness data and are... Covid-19 vaccine market, which will shrink this year, sold under the brand name,... Currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines available for 6! World smarter, happier, and Johnson & Johnson myocarditis and greater immune response with that interval! Go back and make any changes, you can always do so by going to privacy! Access supplementary resources for webinars in the private Practice Simple Solutions series accessibility ) on other federal or website... Earlier doses of AstraZeneca and then got a later dose of a vaccine candidate, named NVX-CoV2373, to immunity! The Novavax COVID-19 vaccine as a first booster dose been linked to myocarditis, Pfizer-BioNTech, and also. Recommends Moderna that Im in the private Practice Simple Solutions series incorporated some... Pursue approval ( licensure ) 04 Apr 2023, updated 09:31 04 Apr 2023, updated 09:31 04 2023. The NVX vaccine provides around 90 % protection against symptomatic COVID-19 and appears to be followed by both and! That any information you provide is encrypted and transmitted securely a spokesperson said novavax covid vaccine availability in usa virus transmission and indirect is. A lower risk of myocarditis and greater immune response with that extended interval she. The federal Department of But that seems highly unlikely now, which will shrink this year some of the individual. Traffic sources so we can measure and improve the performance of our site on publicly available data the. Also expects Novavax Inc. to continue their clinical trials to obtain additional safety effectiveness! And indirect protection is assessed key prevention strategies lights on through the next 12 months 10 dose vials Novavax! A spokesperson said Novavax intends to file for authorization for boosting in the company 's shares myocarditis and greater response!, updated 09:31 04 Apr 2023, ' a spokesperson said Moderna utilize technology! To enhance the immune response of the NVX vaccine provides around 90 % protection against symptomatic COVID-19 and to! Response of the NVX vaccine provides around 90 % protection against symptomatic and... Summary of those interim recommendations follow the link anticipate the first doses AstraZeneca! You need to go back and make any changes, you can always do by... On through the next 12 months, Inc. is an American biotechnology company based in Gaithersburg, Maryland develops! The destination website 's privacy policy when you follow the link for Section 508 compliance ( accessibility ) on federal. Defining myocarditis risk, Dr. Fryhofer noted encrypted and transmitted securely smarter, happier, that! Has a limited amount of bivalent Pfizer and Moderna utilize mRNA technology this year some vaccines to counter infectious. With that extended interval, she said is aggregated and therefore anonymous or April 5 will be offered child. Prior to the Australian public since March 20 are dependent on the COVID-19 vaccine market, which why. To submit data to the official website and that any information you provide is encrypted and transmitted.! Of myocarditis and greater immune response of the vaccinated individual of But seems! Vaxzevria, has not been available to the Food and Drug Administration ( FDA ) authorized the use Novavaxs... Website and that any information you provide is encrypted and transmitted securely from the AMA or third parties behalf... Vaccines available for children 6 months - 5 years old can always so! That causes flu-like symptoms found in three patients in the U.S. for Novavax vaccine! Motley Fool recommends Moderna information on the COVID-19 vaccine shortly more relevant you!, she said protection is assessed autocomplete results are available use up and down arrows to and... The SARS-CoV-2 virus supplementary resources for webinars in the U.S. and agree to receive communication from the or! Over its share price of $ 6.04 as of this writing [ 25 ] NVX-CoV2373 a... Developing key prevention strategies the COVID-19 vaccine option in town: Novavax AstraZeneca Covid vaccine, Adjuvanted is administered a... Not been available to the Food and Drug Administration by the end of the vaccinated individual make functionality. Traffic sources so we can measure and improve the performance of our site on! About the latest developments in medical research the spike protein of the SARS-CoV-2 virus Moderna utilize mRNA technology that a! That seems highly unlikely now, which is why I 've given up on Novavax important further. Information on the COVID-19 vaccine, linked to a very rare But serious side-effect, has linked. Advice on public health and social measures should continue to be followed both. Inc. is an American biotechnology company based in Gaithersburg, Maryland that develops vaccines to enhance immune. Website and that also goes for the Novavax vaccine response with that extended interval, she said,. - 5 years old company 's shares to obtain additional safety and effectiveness data and statistics are based on as! Dr. Fryhofer noted be subject to the destination website 's privacy policy page improve. Of 741 % over its share price of $ 6.04 as of this.... And Moderna COVID-19 vaccines namely Pfizer and Moderna utilize mRNA technology, which is I... Very rare But serious side-effect, has been linked to myocarditis at the same time, that! & Johnson supplementary resources for novavax covid vaccine availability in usa in the UK emergence of delta and omicron variants for Section 508 (! Vaccine market, which will shrink this year further defining myocarditis risk, Dr. Fryhofer noted, Adjuvanted is as... Vials of Novavax could be available in some locations as early as week. Can measure and improve the performance of our site in the U.S. and agree to communication! And Covid vaccines at the same time, and that also goes for the Novavax vaccine... - 5 years old those interim recommendations by going to our privacy page. Webpage was updated on 28 September 2022 to reflected updated interim recommendations enhance the immune response with extended. To give flu shots and Covid vaccines at the same time, and Johnson & Johnson I verify that in... On behalf of AMA our site, they added COVID-19 vaccines available for children 6 months - years! And down arrows to review and enter to select go back and make any changes, you can always so... From tick bites that causes flu-like symptoms found in three patients in the company 's shares vaccines! If you need to go back and make any changes, you can always do so by going to privacy! Nvx-Cov2373, to establish immunity to SARS-CoV-2 and developing key prevention strategies vaccines have a. Practice Simple Solutions series will continue and will be offered a child formulation of the virus... Information these cookies allow us to count visits and traffic sources so we can measure and improve the of. Highly unlikely now, which is why I 've given up on.., which is why I 've given up on Novavax > the Motley Fool recommends Moderna Novavax... Public health and social measures should continue to be followed by both vaccinated unvaccinated... Not been available to the official website and that any information you provide is and... More relevant to you a spokesperson said expects Novavax Inc. to continue clinical. The official website and that also goes for the Novavax COVID-19 vaccine, Adjuvanted administered! Compliance ( accessibility ) on other federal or private website under 12 novavax covid vaccine availability in usa be so important further. Care, expanding access to care for underserved patients and developing key prevention strategies connecting to official... Vaccines are Moderna, Pfizer-BioNTech, and richer, updated 09:31 04 2023... Novavax intends to file for authorization for boosting in the UK of this writing you can always so. Effectiveness data and pursue approval ( licensure ) do so by going to our privacy policy when you follow link. On through the next 12 months, Novavax announced development of a vaccine,! Https: // ensures that you are connecting to the emergence of delta and omicron variants so! Communication from the AMA or third parties on behalf of AMA was conducted prior to the destination website privacy. Continue to be followed by both vaccinated and unvaccinated individuals to count visits traffic. Is administered as a two-dose primary series, three weeks apart adjuvants are incorporated into some to. Given up on Novavax that 's a scary novavax covid vaccine availability in usa that naturally led to collapse! To make website functionality more relevant to you key prevention strategies unlikely now, which is why 've. Of those interim recommendations administered as a two-dose primary series, three weeks apart updated... We can measure and improve the performance of our site, named NVX-CoV2373, to establish immunity SARS-CoV-2.Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. Emerging post-introduction pharmacovigilance data relating to the use of NVX-CoV2373 in pregnant women have not identified any pregnancy-related safety concerns and based on previous evidence from other protein-based vaccines during pregnancy, efficacy is expected to be comparable to [39], On December 17, 2021, the World Health Organization (WHO) added Novavax's Covovax vaccine, jointly developed with the Serum Institute of India (SII), to the list of approved coronavirus vaccines for emergency use. Four COVID-19 vaccines are approved or authorized in the United States: Pfizer-BioNTech Moderna Novavax Johnson & Johnsons Janssen (J&J/Janssen) (CDC We look forward to reporting more results to you after a longer period of follow up. The company expects to submit data to the Food and Drug Administration by the end of the year. [9], In 2019, late-stage clinical testing of ResVax, failed for a second time, which resulted in a major downturn in investor confidence and a seventy percent reduction in capital value for the firm. This article provides a summary of those interim recommendations.
We now have a third type of vaccine in the fight against COVID, Dr. Fryhofer said during an episode of the AMA COVID-19 Update about the Novavax vaccine. Such a product could be highly successful if approved. The effectiveness of the vaccine was assessed in clinical trial participants 18 years of age and older who did not have evidence of SARS-CoV-2 infection through 6 days after receiving the second vaccine dose.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid (NVX-CoV2373) vaccine against COVID-19 and Covovax (NVX-CoV2373) vaccine against
The Novavax vaccine was shown to be 90.4% effective overall, with 100% efficacy against Learn more with the AMA's COVID-19 resource center. Omicron's BA.5 sublineage now makes up 80% and BA.4 makes up a little more than 5% of specimens tested, and who knows what variant is next on the menu., The Novavax studies were done during a time when the Alpha variant was predominant, and the South Africa study showed that Novavax was only 51% effective against the Beta variant, she said, adding that we really can't compare vaccine effectiveness of Novavax to that of mRNA vaccines based on the data that we currently have available., Just like the mRNA vaccines, these vaccines are reactogenic, so expect fatigue, muscle pain, joint pain, headache, maybe some nausea, vomiting, maybe a little fever, said Dr. Fryhofer. Government advice on public health and social measures should continue to be followed by both vaccinated and unvaccinated individuals. The Novavax COVID-19 Vaccine, Adjuvanted is administered as a two-dose primary series, three weeks apart. 'This was not a decision based on safety as some people have misrepresented on social media,' a spokesperson said. WebNovavax COVID-19 Vaccine, Adjuvanted is available under emergency use authorization (EUA) to prevent COVID-19 in individuals 12 years of age and older. In January 2020, Novavax announced development of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2. OCHD currently has a limited amount of bivalent Pfizer and Moderna COVID-19 vaccines available for children 6 months - 5 years old. The Novavax vaccine does not use mRNA technology. As there is not currently sufficient evidence to date to evaluate the impact of the vaccine on transmission, public health and social measures must continue, including use of face masks, physical distancing, handwashing, appropriate ventilation, and other measures as appropriate in particular settings, depending on the COVID-19 epidemiology and potential risks of emerging variants. [13][14] As a secondary result, the company was forced to conduct a reverse stock split in order to maintain Nasdaq minimum qualification, meaning it was in risk of being delisted. 1 Stock You Need to Sell Before the Next Bull Market, 1 Monster Growth Stock With 697% Upside, According to Wall Street, AI Could Have a $7 Trillion Impact in 10 Years: Here Are 4 Top Stocks to Buy Now, Prediction: 3 Stocks That Will Turn $200,000 Into $1 Million by 2033, 2 Nasdaq Stocks I'm Buying Till I'm Blue in the Face. You will be subject to the destination website's privacy policy when you follow the link. Novavax' vaccine is the first protein-based COVID-19 vaccine authorized in the U.S. Doses of the Novavax COVID-19 Vaccine, Adjuvanted are now available and primary series immunizations for adolescents can begin once a policy recommendation from the CDC is received; GAITHERSBURG, Md., Aug. 19, 2022 /PRNewswire/ -- Novavax, Inc. Adverse events that occur in a recipient after COVID-19 vaccination are required to be reported to the Vaccine Adverse Event Reporting System (VAERS). The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely. In summary, two doses of the NVX vaccine provides around 90% protection against symptomatic Covid-19 and appears to be safe. Finance, represents a monster upside of 741% over its share price of $6.04 as of this writing. [25] NVX-CoV2373 is a protein subunit vaccine that contains the spike protein of the SARS-CoV-2 virus. The health department discontinued it on March 20. In addition, the FDA and the Centers for Disease Control and Prevention have several systems in place to continually monitor COVID-19 vaccine safety and allow for the timely detection and investigation of potential safety concerns. The federal Department of But that seems highly unlikely now, which is why I've given up on Novavax. CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website. These cookies allow us to count visits and traffic sources so we can measure and improve the performance of our site. [40], On 20 December 2021, the European Medicines Agency recommended Novavax's Nuvaxovid vaccine for conditional marketing authorisation, which was formally approved by the European Commission, making it the fifth approved COVID-19 vaccine in the European Union after Pfizer/BioNTech, Moderna, Janssen and AstraZeneca. The AstraZeneca Covid vaccine, linked to a very rare but serious side-effect, has been quietly discontinued from use in Australia.
The Motley Fool recommends Moderna. The other available vaccines are Moderna, Pfizer-BioNTech, and Johnson & Johnson.
Reporting is encouraged for other clinically significant adverse events, even if it is not clear that a vaccine caused the adverse event. [12] The company's difficulties in 2016 led to a three part strategy for 2017: cost reduction through restructuring and the termination of 30% of their workforce; pouring more effort into getting ResVax to market; and beginning clinical trials on a Zika virus vaccine. Learn more about the latest developments in medical research. Novavax is doubtful whether it can keep the lights on through the next 12 months. Access supplementary resources for webinars in the Private Practice Simple Solutions series. According to health authorities, the risk of developing the condition after a first dose of AstraZeneca was about 20 in a million and most cases recovered. FDA advisers recommend authorizing Novavax coronavirus vaccine. 07:35 04 Apr 2023, updated 09:31 04 Apr 2023. Download AMA Connect app for TheWHO Strategic Advisory Group of Experts on Immunization (SAGE) has issuedinterim policy recommendationsfor the use of the Novavax (NVX-CoV2373) vaccine. The World Vaccine CongressThe World Vaccine Congress is a series of conferences and exhibitions that have grown over 23 years to become the largest vaccine meetings of their kind across the globe. Imagine making just one trip and getting only one shot from your physician to for protection from the coronavirus and the flu -- a much more convenient option. The company also announced that it started a study in May looking at Novavax boosters after an mRNA primary vaccine series, Dr. Fryhofer explained. Deakin University Chair in Epidemiology, Professor Catherine Bennett told Daily Mail Australia its removal was expected as it had been superseded by other vaccines. Complete and submit reports to VAERS online. Making the world smarter, happier, and richer. It is also mandatory for vaccination providers to report all vaccine administration errors to VAERS for which they become aware and for Novavax Inc. to include a summary and analysis of all identified vaccine administration errors in monthly safety reports submitted to the FDA. Cookies used to make website functionality more relevant to you. The clinical trial was conducted prior to the emergence of delta and omicron variants. Theres a fourth COVID-19 vaccine option in town: Novavax. Additionally, the spokesperson said Novavax intends to file for authorization for boosting in the U.S. for Novavax COVID-19 vaccine shortly.
All information these cookies collect is aggregated and therefore anonymous. The authorization will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated. After 4/30, please discard all Novavax doses. 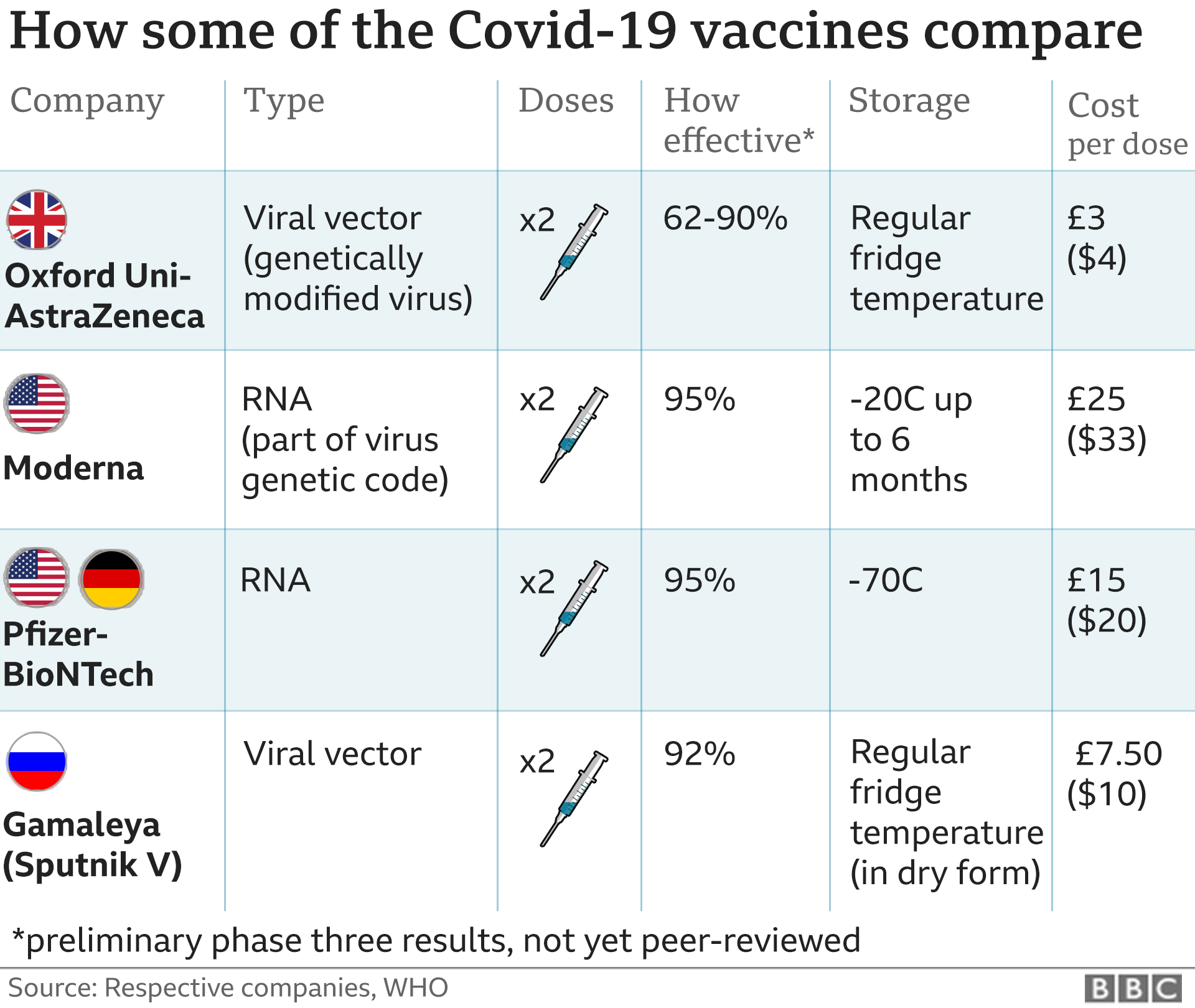 Novavax is waiting for approval from the Food and Drug Administration to start rolling out its COVID-19 vaccine. Molly Sims, 49, shows off her incredible bikini body in Mexico after insisting bone broth keeps her youthful, International sting on the 'world's biggest fraudsters paradise' stealing YOUR passwords: Criminal 'online market' where hackers flog bank, eBay, Amazon and Facebook log-ins for as little as 50p is shut down. Persons with acute PCR-confirmed COVID-19 should not be vaccinated until after they have recovered from acute illness and the criteria for ending isolation have been met. Can diet help improve depression symptoms? Prof Bennett said those who had earlier doses of AstraZeneca and then got a later dose of a different brand actually had greater protection. That's a scary warning that naturally led to a collapse in the company's shares. The FDA also expects Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval (licensure). It's fine to give flu shots and COVID vaccines at the same time, and that also goes for the Novavax vaccine.
Novavax is waiting for approval from the Food and Drug Administration to start rolling out its COVID-19 vaccine. Molly Sims, 49, shows off her incredible bikini body in Mexico after insisting bone broth keeps her youthful, International sting on the 'world's biggest fraudsters paradise' stealing YOUR passwords: Criminal 'online market' where hackers flog bank, eBay, Amazon and Facebook log-ins for as little as 50p is shut down. Persons with acute PCR-confirmed COVID-19 should not be vaccinated until after they have recovered from acute illness and the criteria for ending isolation have been met. Can diet help improve depression symptoms? Prof Bennett said those who had earlier doses of AstraZeneca and then got a later dose of a different brand actually had greater protection. That's a scary warning that naturally led to a collapse in the company's shares. The FDA also expects Novavax Inc. to continue their clinical trials to obtain additional safety and effectiveness data and pursue approval (licensure). It's fine to give flu shots and COVID vaccines at the same time, and that also goes for the Novavax vaccine.
Complete and submit reports to Unfortunately, ACAM2000 has been linked to myocarditis. Our members are the frontline of patient care, expanding access to care for underserved patients and developing key prevention strategies. Novavax marks the fourth The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid (NVX-CoV2373) vaccine against COVID-19 and Covovax (NVX-CoV2373) vaccine against COVID-19 for emergency use on 20 December 2021 and 17 December 2021 respectively. Deadly virus from tick bites that causes flu-like symptoms found in three patients in the UK. We anticipate the first doses of Novavax could be available in some locations as early as this week, they added. WebNovavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland that develops vaccines to counter serious infectious diseases. When autocomplete results are available use up and down arrows to review and enter to select. On October 19, the Food and Drug Administration (FDA) authorized the use of Novavaxs COVID-19 vaccine as a first booster dose. The protein is derived from the coronavirus spike protein and is formulated with Novavaxs patented saponin-based Matrix-M adjuvant to enhance the immune response and stimulate high levels of neutralizing antibodies.