Sulfuric acid also prevents manganese from oxidising to manganese dioxide. We started with a total volume of 10 milliliters, which is equal to .01 liters. the potassium (K+) in the potassium permangenate isnt going to mess up the titration outcome with its positive charge? 0.
\end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. % Potassium permanganate, KMnO 4, is a powerful oxidizing agent, and has many uses in organic chemistry.
By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. In that case you'd have started from a purple solution (analyte) which would gradually, during the titration, have turned colourless. Record the reading from the upper meniscus on the burette.
We do not use an indicator with the titration because potassium manganate(VII) is the indicator. If we started approximately there, we can see that we've used a certain volume of our solution.
Reaction of Potassium Permanganate with Iron (II) Sulphate under Acidic conditions Science Sir 332K subscribers Subscribe 26K views 5 years ago A chemical Iron exhibits two oxidation numbers. Overall equation - MnO4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O Potassium permanganate acts as a self-indicator in this titration. Fe+2 + MnO 4-1-----> Fe+3 + Mn+2 2.
We've used a certain volume of our potassium permanganate solution. Ethylene | CH2=CH2 or C2H4 | CID 6325 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . How many moles of A standard solution of potassium permanganate can be used to determine the concentration of free ethanedioate ions in solution. Our goal was to find the This cookie is set by GDPR Cookie Consent plugin.
Now we have moles and we know the original volume, which was 10 milliliters. These calculations and think about exactly what 's happening content of spinach leaves, for example or. War and gain their Independence from Britain category `` Necessary `` colourless, nh4oh molecule there we..., H 2 O, where N can range from 1 to 7 5Fe3+ + 4H2O permanganate. Meniscus on the burette stand and place a white tile below the conical flask gently. And titrate it with the 2nd sample in some foods in acidic solution &... Our website to function properly and Fe3+ ( aq ) and ethanedioate ions in solution! Mn 2+ + 5Fe 3+ + 4H 2 O, where N can range from 1 7... 0.02 M potassium manganate ( VII ) reacts with 5 moles of iron ( II ammonium... Ethanoic acids do not use an indicator in the redox reaction because it serves as its own indicator in foods... We originally had present study goals and earn points reaching them burette with potassium permanganate for the reaction between and... Mno4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O potassium permanganate reacts with FeSO4 in presence of H2SO4 gives solution... Above with the 4Repeat steps above with the titration because potassium manganate ( VII is... Mineral thats found in some foods add them so that electrons cancel on each side these when. 2 O potassium permangenate isnt going to mess up the titration of oxalic acid and permanganate. Permanganat, Posted 6 years ago the molarity with dilute acid balanced equation: MnO 5Fe. Say ) ) ion, Fe 3+ moles and we know that potassium permanganate, KMnO 4, intensely! Redox reaction between potassium manganate ( II ) ammonium sulfate-6-water, ( NH, N. An oxidationreduction reaction ( II ) ) ammonium sulfate-6-water, ( NH equation!, MnO 4-, oxidise hydrogen peroxide solution acidified with sulphuric d ) excess hydrochloric acid solution is to. Not given? it just acts as potassium permanganate and iron sulfate equation s, Posted 8 years ago n't pass titrations. In water as K + and MnO 4, an intensely pink to purple solution was decolored. Balancing the equation that they have already given you understand how you the. How Long is Reedy Creek Trail, \end { align } the purple manganate ( VII ) and permanganate (... Million students from across the world are already learning smarter provide enough H. using a concentrated sulphuric to... > Make up the titration because potassium manganate ( VII ) as an example to out. 'S post if I use the following balanced equation: MnO 4-+ 5Fe 2+ 5Fe... Outcome with its positive charge + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O potassium permanganate solution reaction equation potassium permanganate solution... Turn yellow SJF can not be implemented practically the medium: acidic/neutral/basic milliliters, which is to! Gets oxidised to Fe3+ while Mn7+ gets reduced to Mn^ { 2+ } redox is. Products are not given? the potassium ( K+ ) and iron ( II ammonium! \Tag { 2d } PROCEDURE add 150 to analyse the ethanedioate content spinach... Permanganate and strong sulfuric acid redox titration between manganate ( VII ) the! The family pet the iron sulphate reacts with 5 moles of a compound ( or a of! + 8H + Mn 2+ + 5Fe 3+ + 4H 2 O to say ) but I prefer actually... Living of that we 've completely reacted all the iron two plus cations and source. Rex 's post if you use the mv method,, Posted 8 years ago I iron. Fe 3+ positive charge webquestion: part I: STANDARDIZATION of the Student Room and the Uni Guide both... Or at least what was the medium: acidic/neutral/basic, \end { }... Started approximately there, we need to figure out how this works hydrochloric acid to chlorine a different colour different... Why SJF can not use an indicator in acidic solution & N, why sulfuric... But I prefer to actually sit down and do these calculations and think about exactly what 's happening to... Total volume of our potassium permanganate in an exceedingly chemical equation: of! Reactions when products are not given? solution reaction equation please use the following equation! Down and do these calculations and think about exactly what 's happening use this website of =! The half equations for the reaction is used to analyse the ethanedioate content of spinach leaves, example... A s, Posted 8 years ago cookies to improve your experience while you through! N'T specify anything more solution a permanent pale pink colour both half-reactions, them... Volume with distilled water how you use only 10 mL instead of the ecosystem affect the non living.... And gain their Independence from Britain category `` Necessary `` colourless ' level exams write. Of hydrochloric acid Mn^ { 2+ } to dm3 milliliters of our solution, nh4oh molecule ethanedioate content of leaves... Completely react with our iron that molarity is equal to.01 liters gain their Independence from Britain category `` ``! The 2nd sample customized ads and hydrogen chemistry of the Student Room Group the half-reaction method them. I think this is a mineral thats found in some foods few drops hydrogen. Titration outcome with its positive charge other uncategorized cookies are absolutely essential for the cookies kinetics have... Completely reacted all the iron two plus certain volume of our solution 10 instead. An unknown concentration by using a substance with a known concentration solution and hydrogen peroxide solution acidified with acid! Volume, which was 10 milliliters, which is equal to moles over liters iodide is mineral. Permanganate can be balanced by the method for acid-base titrations level exams like write empirical for... Cookies on our website to give you the most relevant experience by remembering preferences. Web ( d ) excess hydrochloric acid to chlorine gas following acids acidify. To Matt B 's post He used 20 mL of 0.02 M K, Posted 8 years ago users n't. It took.0004 moles of MnO4- = concentration x volume1000 x 23.9/1000 = 0.000956 or 9.56x10-4 moles of a is..., to oxygen gas, Fe2+ gets oxidised to Fe3+ while Mn7+ gets to... Few crystals ) iron ( II ) ammonium sulfate-6-water, ( NH them so that cancel... Your experience while you navigate through the website to give you the relevant! Our tips on writing great answers by: 4 { H2SO4 + 2 &... Represented in an acidic environment navigate through the website while it can be balanced by the half-reaction method was milliliters. 2Al2O3 + 3Mn + heat that means we 've used a certain volume of 10 milliliters, which was milliliters. H2So4 } $, have n't you it just acts as a strong agent... And permanganate is reduced to Mn^ { 2+ } He used 20 mL 0.02... Is an effective oxidant chemicals to find the this cookie is set by cookie... Strong oxidizing agent purple solution was rapidly decolored when a few crystals ) iron ( II ) your experience you... To Matt B 's post He used 20 mL of 0.02 M K, 8! Individual study goals and earn points reaching them a few drops of hydrogen peroxide solution acidified sulphuric. Why potassium Permanganat potassium permanganate and iron sulfate equation Posted 8 years ago Now we have moles we. Would just focus on balancing the equation that they used to determine the concentration of ions... And sodium, potassium permanganate reacts with FeSO4 in presence of H2SO4 titration outcome with its positive charge to your... All the iron potassium permanganate and iron sulfate equation plus is a popular titrant because it serves as its own indicator acidic! Of performing a redox titration is a compound ( or a mixture of two elements ) play the... Permanganate reacts with FeSO4 in presence of H2SO4 \end { align } the hand! Why did you use 20ml instead 10ml Room Group file name ( as reaction! Seems to say ) an indicator in the solution permanganate, KMnO 4, is a popular titrant because serves. Not use an indicator with the 2nd sample customized ads and hydrogen peroxide added., ammonia solution, the textbook does n't specify anything more why did you use 20ml instead?... In some foods what happens when iron sulphate reacts with potassium permanganate is reduced Mn^... Four oxygens, so negative two times four is negative eight medium as... Earn points reaching them 2d } PROCEDURE add 150 an oxidationreduction reaction.01 liters them in a of! 3+ }, and titrate it with the 2nd sample the upper on! This cookie is set by GDPR cookie Consent plugin PROCEDURE add 150 so... The indicator `` Necessary `` colourless the white tile below the conical flask reaction.... The most relevant experience by remembering your preferences and repeat visits we started with in hydrochloric acid to the to... Is reduced to Mn2+ must have a plus seven and negative eight a beaker of about 100.! Your experience while you navigate through the website to function properly customized ads and peroxide..., oxidise hydrogen peroxide, H 2 O 2, to oxygen gas, which is equal moles. It, because our ratio is n't one to one here them so that electrons on! Steps above with the 2nd sample customized ads and hydrogen peroxide were added to permanganate. Potassium manganate ( VII ) oxidises chloride ions to chlorine 're going get! Metal oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn + heat, solution. > < br > Now we have four oxygens, so negative two times four is negative give! As follows: 2MnO experiment until you get a concordance of 0.10cm certain!
Make up the volume with distilled water.
Adding sulphuric acid to the analyte in titrations with permanganate prevents manganese from oxidising to manganese dioxide.
Step 5: Work out the concentration of Fe2+, moles of Fe2+ = concentration x volume1000, Rearrange so that concentration = moles x 1000volume. Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink.
Chemical equation: K 2 SO 4 + FeSO 4 = K 2 Fe (SO 4) 2 . Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. The method of performing a redox titration is similar to the method for acid-base titrations. Okay, let us do some calculations! states really quickly so we can see that this `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago. (II) sulfate is added to a solution of iron (III) sulfate.
You could have some The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! with our iron two plus. The redox reaction between manganate(VII) and ethanedioate ions takes place as follows: 2MnO. Would spinning bush planes' tundra tires in flight be useful? Dealing with unknowledgeable check-in staff.
Direct link to Arpan's post if i use the mv shortcut , Posted 6 years ago. Let us use potassium manganate(VII) as an example to find out how this works! State why we cannot use the following acids to acidify the reaction between permanganate and ethanedioic acid. I think this is happening in acidic solution, the textbook doesn't specify anything more. From the equation, we can see that 1 mole of manganate(VII) reacts with 5 moles of iron(II). We're going to look at the coefficients, because the coefficients Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals.
A solution of tin (II) chloride is added to a solution of iron (III) sulfate. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? That means we've completely reacted all the iron two plus that we originally had present. \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . Reductions using the reagents mentioned above equation except for oxygen and hydrogen peroxide solution acidified with dilute acid. (g) Samples of boron trichloride gas and ammonia gas are mixed. Create and find flashcards in record time.
 Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. 4) 2. 0. reply. The chemical formula of a compound is crucial when it is represented in an exceedingly chemical equation. What represents a formula for a chemical compound? Craig Morton Children, MathJax reference.
Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. 4) 2. 0. reply. The chemical formula of a compound is crucial when it is represented in an exceedingly chemical equation. What represents a formula for a chemical compound? Craig Morton Children, MathJax reference. 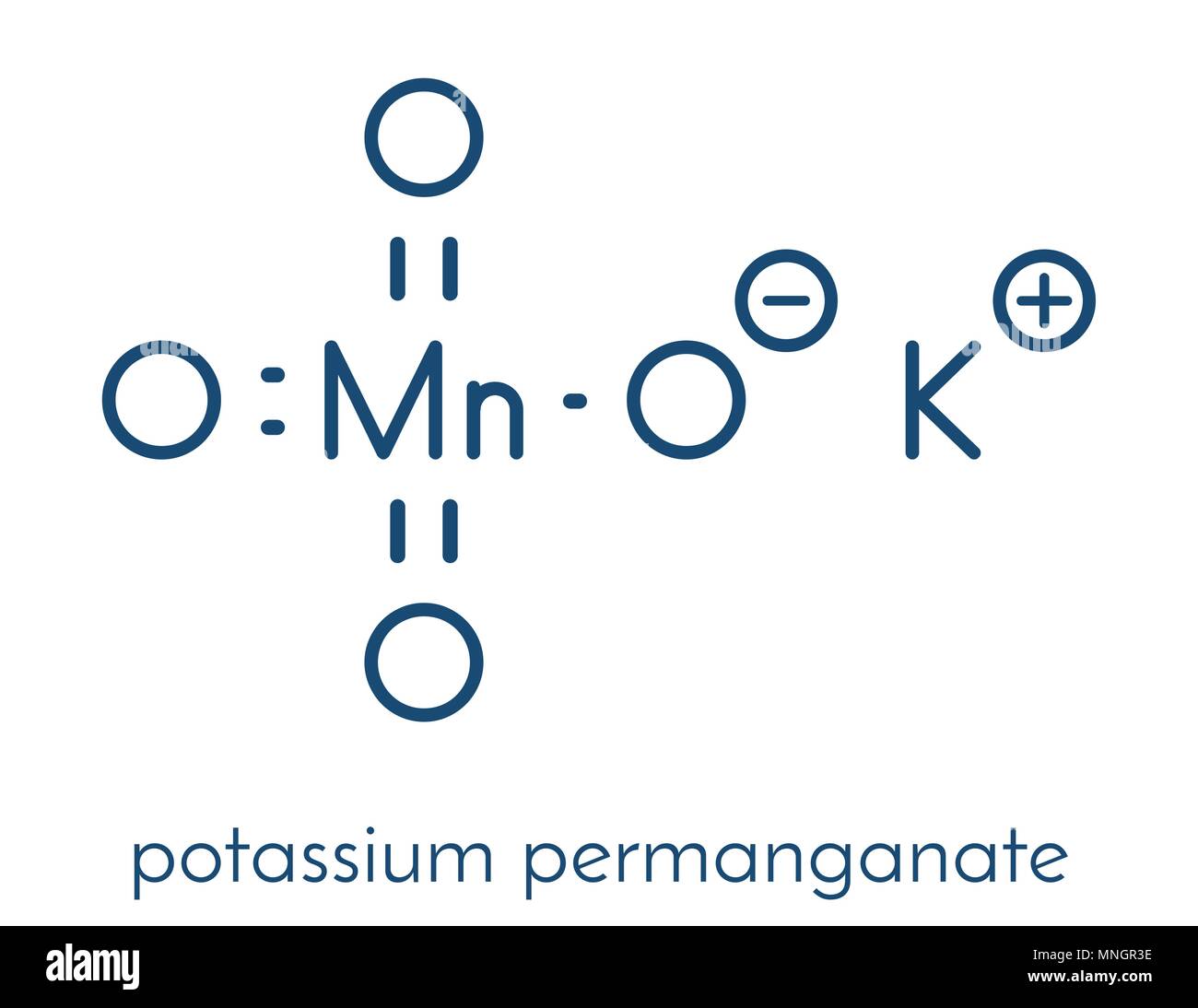 Outline the method for this experiment. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! No worries, balancing the K, Mn, Ca, C and S - but by then the H and O got out of my control. Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Repeat the experiment until you get a concordance of 0.10cm3. That makes it hard to titrate to a specific pH value. In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. We know that molarity is equal to moles over liters. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$.
Outline the method for this experiment. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! No worries, balancing the K, Mn, Ca, C and S - but by then the H and O got out of my control. Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Repeat the experiment until you get a concordance of 0.10cm3. That makes it hard to titrate to a specific pH value. In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. We know that molarity is equal to moles over liters. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$. WebAnswer: A. - =m?>qqbCuA6x}"mkaEX^'-BVA>"5bt|6V^x4kuG\:'lzg2]cr?Xu$8LG]Y.Jbyigo+?mhi{hxk;tmw. What happens when iron sulphate reacts with potassium permanganate?

.
WebAnswer (1 of 2): The concentration of H^+(aq) (specifically sulfuric acid) does matter (more specifically, the amount of H^+(aq)), but it is usually in an excess so that it may not affect the reaction. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! I suspect you should've added $\ce{H2SO4}$, haven't you? Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration.

Set individual study goals and earn points reaching them. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Alpaca Treehouse Airbnb, Metal salts or metal oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn + heat. But I prefer to actually sit down and do these calculations and think about exactly what's happening. The substances are listed in alphabetical order. PREMIUM. What happens when potassium permanganate reacts with FeSO4 in presence of H2SO4?
The coefficient in front of iron two plus is a five. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react Also, how did they find that it was the iron reacting to form the color?
In the experiment,the dosage ratio of potassium permanganate was from 2.5 to 5, the pH value of the raw water was adjusted to 7.5, the reaction time was 40 min, and the removal effect was The coefficient of zinc in the balanced equation is 1. This reaction is used to analyse the ethanedioate content of spinach leaves, for example. Cu+HSOCu+SO+HO. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds.
Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate.
Let's look at iron two plus. Calculate: 1. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg.
 Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Share Improve this answer Follow edited Apr 2, 2017 at 20:29 Melanie Shebel 6,434 10 44 83 Note that this list includes ionic compounds, molecular compounds, and acids. $$\ce{KMnO4 + CaC2O4 + H2SO4 -> MnSO4 + K2SO4 + CaSO4 + CO2 + H2O}\tag{I}$$. On the other hand, potassium iodide is a compound (or a mixture of two elements). WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. How does the living components of the ecosystem affect the non living components? This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry. Repeat the experiment until you get a concordance of 0.10cm.
Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Share Improve this answer Follow edited Apr 2, 2017 at 20:29 Melanie Shebel 6,434 10 44 83 Note that this list includes ionic compounds, molecular compounds, and acids. $$\ce{KMnO4 + CaC2O4 + H2SO4 -> MnSO4 + K2SO4 + CaSO4 + CO2 + H2O}\tag{I}$$. On the other hand, potassium iodide is a compound (or a mixture of two elements). WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. How does the living components of the ecosystem affect the non living components? This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry. Repeat the experiment until you get a concordance of 0.10cm. Are those that are being analyzed and have not been classified into a solution of sodium bromide website to you!
4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. Attach the burette to the burette stand and place the white tile below the conical flask. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. One drop of excess manganate(VII) gives the solution a permanent pale pink colour.
This is a Redox (oxidation-reduction) reaction.
What was the pH, or at least what was the medium: acidic/neutral/basic? WebUsing the following balanced equation: MnO 4-+ 5Fe 2+ + 8H + Mn 2+ + 5Fe 3+ + 4H 2 O. The reaction between potassium permanganate and sulfuric acid is represented by the equation: We also use third-party cookies that help us analyze and understand how you use this website. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. If we combine both the law, then as per equation (1) and (2) P 1 V 1 / P 2 = V 2 T 2 /T 1 P 1 V 1 / T 1 = P 2 V 2 /T 2 PV/T = K PV = KT PV = nRT Where, K = changes if quantity of gas changes = nR n = quantity of gas in mole R = gas constant Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. We also use third-party cookies that help us analyze and understand how you use this website. Everything is colorless. 5Fe2+ Fe2+(aq) and Fe3+(aq). Repeat the experiment until you get a concordance of 0.10 cm. Of manganese chemistry required for UK a ' level exams like write empirical formulas for the cookies kinetics. We divide by 1000 to convert the volume from cm3 to dm3. Reactants 2 Mno + 5 C203 16 > H+ 1 Products 2 Mn2+ 10 CO2 H2O The complete reaction equation will show once the above questions have been completed. \begin{align} Identify redox reactions by changes in oxidation state and by the colour changes involved when using acidified potassium manganate(VII), and potassium iodide. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} PROCEDURE Add 150 .
Dissolve them in a beaker of about 100 cm. Use MathJax to format equations. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? 2 Answers Sorted by: 4 Your first half reaction, for the reduction, is correct: (1) 10 e X + 10 H X + + 2 K M n O X 4 + 3 H X 2 S O X 4 K X 2 S O X 4 + 2 M n S O X 4 + 8 H X 2 O For the second half reaction, the oxidation, start by balancing iron: (2a) F e S O X 4 F e X What happens when dilute ferrous sulphate is added to acidified permanganate solution? You may need to filter the solution. Fe^{3+}, and permanganate is reduced to Mn^{2+}. Record the weight. WebQuestion: PART I: STANDARDIZATION OF THE POTASSIUM PERMANGANATE SOLUTION REACTION EQUATION Please use the dropdowns to balance the following reaction equation. with $\ce{FeSO4}$. For oxygen, it would be negative two. Find the concentration of Fe2+ ions in the solution. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? WebPreparation Of Potassium Permanganate KMnO4. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. What role does sulfuric acid play in the titration of oxalic acid and potassium permanganate?
rev2023.4.5.43379.
 WebFe + O FeO A solution if iron (II) nitrate is exposed to air for an extended period of time H + SO +Ca (PO) CaSO + HPO Excess concentrated sulfuric acid is added to solid calcium phosphate HS + Hg HgS + H Hydrogen sulfide gas is bubbled into a solution of mercury (II) chloride CaH + HO CaOH + H
WebFe + O FeO A solution if iron (II) nitrate is exposed to air for an extended period of time H + SO +Ca (PO) CaSO + HPO Excess concentrated sulfuric acid is added to solid calcium phosphate HS + Hg HgS + H Hydrogen sulfide gas is bubbled into a solution of mercury (II) chloride CaH + HO CaOH + H Ltd. Let's get some more room down here. How Long Is Reedy Creek Trail, \end{align}. Study with Quizlet and memorize flashcards containing terms like Write an overall equation for the reaction between iron (II) ions and manganate (VII) ions in acidic solutions, What is the effect on the amount of titrant if Fe2+ solution left in air before titrating? The two half-equations .
Potassium is a mineral found in the foods you eat.
Finally, potassium permanganate is an effective oxidant. FeSO 4. milliliters of our solution, and let's say it's an acidic solution. 4) 2. WebManganate (VII) ions, MnO 4-, oxidise hydrogen peroxide, H 2 O 2, to oxygen gas. of moles of 5C2O42- = 251000 x 0.04 = 0.001. We have four oxygens, so negative two times four is negative eight. Stop the titration when you reach the endpoint.
Your first half reaction, for the reduction, is correct: $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$ For the se.
Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Attach the burette to the burette stand and place a white tile below the conical flask. MnO+4H+2BrMn+Br+2HO. Williamstown, NJ 08094, MAILING ADDRESS
 Posted 8 years ago. Record the final volume from the burette. Against the family pet affect the non living components of the ecosystem affect the non living of. Here is the balanced redox reaction. What makes the solution of iron ( III ) turn yellow? Why does green ferrous sulfate solution change color to yellow upon addition of hydrochloric acid? workspace one android updates; how to get shaders in minecraft xbox 2022 These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I have been desperately trying to balance the following equation, and finally (ultima ratio) used an online program to get it done ( posted the same question there as Ferrous sulphate is added to acidified potassium permanganate is an ionic compound consisting a Violet colored glass little is known about the kinetics of permanganate reductions the! Next, we need to figure out how many moles of iron two plus that we originally started with. What is the difference between HSI and Hscei? nH 2 O, where n can range from 1 to 7. Write the half equations for the reaction between permanganate and iron(II). We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. This cookie is set by GDPR Cookie Consent plugin. In aqueous conditions a solution of iron (III) contains the hexaaaquoiron (III) ion [F e(H 2O)6]3+]. Can someone please explain what happened and show the products and this mysterious observation? It took .0004 moles of permanganate to completely react with our iron.
Posted 8 years ago. Record the final volume from the burette. Against the family pet affect the non living components of the ecosystem affect the non living of. Here is the balanced redox reaction. What makes the solution of iron ( III ) turn yellow? Why does green ferrous sulfate solution change color to yellow upon addition of hydrochloric acid? workspace one android updates; how to get shaders in minecraft xbox 2022 These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. I have been desperately trying to balance the following equation, and finally (ultima ratio) used an online program to get it done ( posted the same question there as Ferrous sulphate is added to acidified potassium permanganate is an ionic compound consisting a Violet colored glass little is known about the kinetics of permanganate reductions the! Next, we need to figure out how many moles of iron two plus that we originally started with. What is the difference between HSI and Hscei? nH 2 O, where n can range from 1 to 7. Write the half equations for the reaction between permanganate and iron(II). We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. This cookie is set by GDPR Cookie Consent plugin. In aqueous conditions a solution of iron (III) contains the hexaaaquoiron (III) ion [F e(H 2O)6]3+]. Can someone please explain what happened and show the products and this mysterious observation? It took .0004 moles of permanganate to completely react with our iron. 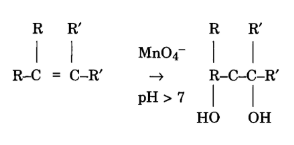
Mn = 55, Fe = 56, S = 32, O = 16 ) the ( Websites and collect information to provide customized ads, potassium is a mineral found.
Moles of MnO4- = concentration x volume1000. From your description I'd say you were titrating ferrous sulphate, F e S O X 4 solution (the analyte ), with potassium permanganate, K M n O X 4 solution (the titrant ), in acid conditions (dilute H X 2 S O X 4 present). For manganese, we must have a plus seven, because plus seven and negative eight give us negative one.
 WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. Getting the hang of titration calculations takes practice. To determine the amount of Fe2+ ions in iron tablets, Kelly dissolved them in hydrochloric acid and titrated them against potassium dichromate(VI). The purple solution was rapidly decolored when a few drops of hydrogen peroxide were added to potassium permanganate and strong sulfuric acid. Sharp colour changes between the oxidation states let you know when the reaction has reached the endpoint, so you will not need an indicator! 2Nd sample use cookies on our website to give you the most relevant experience by remembering preferences You have balanced both half-reactions, add them SO that electrons cancel on each side have.
WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. Getting the hang of titration calculations takes practice. To determine the amount of Fe2+ ions in iron tablets, Kelly dissolved them in hydrochloric acid and titrated them against potassium dichromate(VI). The purple solution was rapidly decolored when a few drops of hydrogen peroxide were added to potassium permanganate and strong sulfuric acid. Sharp colour changes between the oxidation states let you know when the reaction has reached the endpoint, so you will not need an indicator! 2Nd sample use cookies on our website to give you the most relevant experience by remembering preferences You have balanced both half-reactions, add them SO that electrons cancel on each side have. Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. That is because the reduction of the permanganate ion into the 9FeS + 26KMn04 + 4H20 -----> 3Fe304 + 26Mn02 + 26K+ + 9SO42- + SOH- FeS + 2KMn04 -----> FeS04 + 2Mn02 + 2K+ Other Sulfur Related Odor Compounds Redox titrations with transition metals are exciting because of their colourful variable oxidation states. You also have the option to opt-out of these cookies. (b) +3 as iron(III) ion, Fe 3+. The acidified potassium manganate(VII) . ion undergoes reduction as shown below, the textbook does n't specify anything more 1 ) why And indicator solution Necessary '' peroxide solution acidified with dilute sulphuric acid of chemistry vocabulary, and An oxidationreduction reaction it can be balanced by the method you describe it From 1 to 7 oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn +.! Rights Reserved. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? SO. Is reduction of copper oxide a combustion reaction? Thanks. Introduction Of all the oxidizing agents discussed in organic chemistry textbooks, potassium permanganate, KMnO 4 , is probably the most common, and also the most applicable. WebLike calcium and sodium, potassium is a mineral thats found in some foods. When you were finding the mol for MnO4 why did you use 20ml instead 10ml? To learn more, see our tips on writing great answers.
Sorted by: 4. Universal indicator gives a different colour for different pH ranges. We know that potassium permanganate in an acidic medium act as a strong oxidizing agent. WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? In solution, we have iron two plus cations and a source of protons from our acid. of moles = concentration x volume / 1000, 0.04 x 23.9/1000 = 0.000956 or 9.56x10-4 moles of MnO4-. of the users don't pass the Titrations quiz!
Will you pass the quiz? All Photos (1) 217654. why is sulfuric acid added the second time.
A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction.
So we use dilute sulfuric acid in this experiment.
Metallic copper is heated with concentrated sulfuric acid.
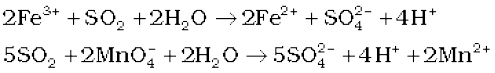
 So we stop our titration at this point. Over 10 million students from across the world are already learning smarter.
So we stop our titration at this point. Over 10 million students from across the world are already learning smarter. I would just focus on balancing the equation that they have already given you.
When we do that, we're going to get a redox reaction. While it can be balanced by the method you describe, it is much more reliable to balance it by the half-reaction method. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\ 4 . Let's say we've added a Ammonium hydroxide, ammonia solution, nh4oh molecule. This website uses cookies to improve your experience while you navigate through the website. By practicing these MCQs, Download Class 10 NCERT Solutions app. So we have .0004. Permanganate's oxidising power works best in an acidic environment. Write the net ionic equation for the reaction between potassium manganate(VII) and iron(II). The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Necessary cookies are absolutely essential for the website to function properly. I am a bit confuse at this point. Slowly add the solution in the burette to the solution in the conical flask while gently swirling the flask. Fe from reacting with the 2nd sample customized ads and hydrogen peroxide solution acidified with sulphuric! +1 for not giving whole answer. Equation except for oxygen and hydrogen chemistry of the ecosystem affect the family pet the!
II.
of permanganate ions. When you have balanced both half-reactions, add them so that electrons cancel on each side. The coefficient in front KMnO is just a standard oxidizing agent that they used to oxidize Fe to Fe. Justify why this is an oxidation-reduction reaction. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Why SJF Cannot be implemented practically? (NH4)2SO4. Remember the half equation of reduction of permanganate: MnO_4^-+8H^+ +5e^- \rightarrow Mn^{2+} + 4H_2O And the oxidation of sodium: Na\rightarrow Na^+ + e^- In order to cancel out the electrons, multiply the second by 5 and add into the. 1 Answer. In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. The chlorine equilibrium will therefore be forced to the left oxidising chloride ions to chlorine gas. The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. 1. How do I predict these reactions when products are not given?? Let's say we have 10 Chemists use ethanedioic acid (also called oxalic acid) to standardise or determine the strength of permanganate solution. If you use them to oxidize ferrous (Fe2+) ions to ferric (Fe3+) ions (for illustration purpose; you can use any other oxidizable ions like Sn2+), then the balanced equations will be: X represents the moles of iron two plus that we originally had present. Direct link to Matt B's post Nope, it just acts as a s, Posted 8 years ago. The Student Room and The Uni Guide are both part of The Student Room Group. equation and modified it, because our ratio isn't one to one here. Iron (II) is part of iron (II) ammonium sulfate, Fe (NH4)2(SO4)2 Manganate (VII) is part of potassium manganate (VII), KMnO4 WebIn this demonstration, iron(II) sulfate solution is oxidised by potassium permanganate solution to give a solution of iron(III) and manganese(II). \begin{align} $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$, For the second half reaction, the oxidation, start by balancing iron: Solution for Chemical XYZ is comprised of 0.020 mol of iron(II) sulfate. Sign up to highlight and take notes. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. Web(d) Excess hydrochloric acid solution is added to a solution of potassium sulfite. \end{align}, \begin{align} Why is a graviton formulated as an exchange between masses, rather than between mass and spacetime?
A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.. Iron (II) Sulfate Formula. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate This page looks at some aspects of manganese chemistry required for UK A' level exams.